| Issue |
Sci. Tech. Energ. Transition
Volume 79, 2024
Synthesis and characterisation of porous materials for clean energy applications
|
|
|---|---|---|
| Article Number | 84 | |
| Number of page(s) | 14 | |
| DOI | https://doi.org/10.2516/stet/2024050 | |
| Published online | 23 October 2024 | |
Regular Article
Investigating the crystallite morphologies and aggregation in boehmite powders: a combined analysis of two- and three-dimensional electron microscopy with X-ray diffraction
1
Institut de Physique et Chimie des Matériaux de Strasbourg (IPCMS), UMR 7504 CNRS – Université de Strasbourg, 23 Rue du Loess, 67200 Strasbourg, France
2
IFP Energies nouvelles (IFPEN), Rond-Point de l’Echangeur de Solaize, BP 3-69360, Solaize, France
3
Physicochimie des Electrolytes et Nanosystèmes interfaciaux Laboratory (PHENIX), CNRS and University Pierre et Marie Curie, 75252 Paris cedex 5, France
4
IFP Energies Nouvelles (IFPEN), 1 et 4 avenue de Bois-Préau, 92852 Rueil-Malmaison Cedex, France
* Corresponding author: virgile.rouchon@ifpen.fr
Received:
15
January
2024
Accepted:
25
June
2024
Boehmite (AlOOH) is considered as an important precursor for γ-Al2O3, which when calcinated undergoes topotactic transformation to form the latter. Alumina has extensive applications in fields such as catalysis, abrasives, and cosmetics among others. Boehmite falls under the category of hierarchical structures whose structural and textural properties are a result of its compositional and porous hierarchy. Although research has been carried out extensively to understand the complete representation of its structure, a true morphological model is an important key to understanding and fully explaining its transport properties during catalytic processes. 3D electron microscopy helps us to dive deeper into the different hierarchical entities of boehmite, bridging the gaps between the models and assumptions made using some more traditional characterization techniques. We present here a deep insight into the structural and morphological parameters of several commercial boehmites using 3D transmission electron microscopy. Through the extraction of quantitative descriptors pertaining to hierarchical entities and subsequent comparison with bulk analyses, precise and comprehensive information regarding these microstructures can be obtained. The results of our study indicate that boehmite grades, which appear to be identical in terms of their grades, display discrepancies in the uniformity of particle sizes. Moreover, diverse platelet interactions result in varying types of pores in these grades. Furthermore, it has been observed that the interfacial interactions among various crystallographic planes exhibit variations across different specimens, thereby contributing to the distinctive compositions within the aggregates. The variation in aggregates of different boehmite grades is also reflected in the combination of four distinct quantified morphologies.
Key words: Boehmite / Nanocrystal / Electron tomography / Particle size distribution / Crystal morphology / Aggregation
© The Author(s), published by EDP Sciences, 2024
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
1 Introduction
From the earliest days of industrial catalysis, boehmites have been used as a support for the production of heterogeneous catalysts and as precursor materials for the synthesis of alumina in various industries [1–5]. Like other nanoparticulate oxyhydroxides, various factors such as pH, temperature, and time influence the morphology of the final boehmite. The crystal structure of boehmite (AlOOH) is distinguished by double layers, in which six oxygen (O) atoms, thereby creating an AlO6 octahedron, encompass an aluminum (Al) atom positioned at the center. The hydroxyl groups are formed through the bonding between the oxygen (O) atom located on the periphery of the double-layered AlO6 octahedrons and a hydrogen (H) atom. The parallel alignment of adjacent octahedrons in an organized layer structure is a result of the hydrogen bonding interactions among the hydroxyl groups (-OH). This ordered arrangement of layers holds considerable significance in determining the performance features of boehmite [6].
The modification of pH conditions, acid radical ions, surfactants, and solvents results in the alteration of interactions and behaviors of Al, O, and H species, ultimately leading to the formation of boehmite with unique structural features. In a recent study by Zhang et al. [7], the effect of important factors such as pH on the morphology and structure of boehmite was investigated, and it was concluded that the size and morphology of boehmite nanoplatelets vary between hexagonal and rhombic depending on the concentration and choice of precursors, synthesis temperature and pH. Moreover, the impact of the distribution of protonated and deprotonated amphoteric sites on the growth of AlOOH nanocrystallite was also explained. The growth of boehmite nanocrystallites undergoes a transformation from simple planar shapes to hexagonal platelets, and eventually to rhombic platelets as the pH values increase. The studies by Panias et al. [8] yielded valuable insights regarding the diverse hydroxo aluminate complex ions that exist under different pH conditions. Under neutral or basic pH conditions, it is common for boehmite crystals to exhibit growth along the [100] and [001] crystallographic orientations. This growth pattern leads to the development of 2D structures that resemble flakes or platelets, as reported by Jiao et al. [9]. The observed behavior can be explained by the relatively stronger covalent bonding interactions that occur between O and Al atoms along the in-layer crystallographic axes (a and c), in comparison to the hydrogen bonding interactions that occur between the double layers of octahedra along the b axis. The binding affinity between Al3+ ions and other functional groups, as well as the interfacial tensions between the oxide surface and the surrounding solution, are both influenced by pH values [10, 11]. The impact of different pH values on the distribution of aluminum ion species in the crystallization environments and its consequent influence on the morphology of boehmite were also observed in a study conducted by Adschiri et al. [12]. Under conditions of low pH, positively charged aluminum species, namely Al(OH)2+ and Al(OH)2 +, were found to selectively adsorb onto the negatively charged surface of the boehmite particle. The formation of boehmite with a one-dimensional morphology was observed as a consequence of the selective adsorption of these aluminum ions and the elongated structures of the resulting boehmite were found to be aligned along a single axis.
Several other studies [13–15] also investigate the influence of various conditions such as the acidity of the solution, temperature, and type of anion on the size and shape of these synthesized boehmite nanoparticles. For example, Chiche et al. [16] examined the effect of pH alongside the carbon chain length of polyols where the morphology of the resulting boehmite varied according to the number of polyol carbons and pH. In acidic conditions, boehmite forms single-dimensional nanorods whereas in basic conditions, the resulting structure is three-dimensional nanoplates or often nanocubes. Boehmite particle growth is controlled in shape by acid radical anions as well, which include NO3 –, SO4 2–, Cl–, and CH3COO–. Selective adsorption onto specific crystallographic axes, such as (010) and (001), results in one directional growth along the [100] axis [9]. The adsorption efficacy of these anions is dependent upon their ratio of charge to size, whereby higher ratios tend to promote adsorption. During hydrothermal synthesis routes to form AlOOH, the anions undergo a gradual desorption phenomenon and are subsequently substituted by water molecules which in turn leads to a reduction in their shape-directing capabilities during the Ostwald ripening process [17].
The systematic manipulation of synthesis parameters like pH, temperature, and surfactants can feasibly adjust the growth rate of various crystallite facets thus controlling and tuning the resulting morphology. Certainly, when undertaken correctly, bench-scale synthesis or experimentations can give an accurate forecast of eventual particle size, shape, and morphological aspects but the factors involved during a bulk industrial production may lead to more heterogeneous morphologies of the resulting nanocrystals [18, 19]. The present study corresponds to the examination of the size, shape, and aggregation configuration of crystalline particles in four different boehmite grades that were produced through industrial synthesis routes. Local characterization techniques such as X-ray diffraction (XRD) and classical transmission electron microscopy (TEM) are utilized in conjunction with 3D imaging by electron tomography. The investigation of the interactions among crystallites is also carried out via electron tomographic imaging of aggregates. This investigation aims to obtain a thorough comprehension of the microstructural fingerprint of different industrial boehmite grades, by a combination of diverse analytical methodologies.
2 Experimental
The present investigation primarily utilizes XRD for the identification of unique crystallographic characteristics. Furthermore, we employ both 2D- and 3D-TEM techniques to quantitatively evaluate the dimensions, morphology, and interfacial characteristics of the diverse platelet structures.
2.1 Materials
This study selected four grades of boehmite (Table 1) based on their relatively larger average crystallite size of 40 nm, which facilitates the analysis of individual crystallites in both two and three dimensions in the TEM. The chemical composition of the grades has been explicitly defined, encompassing the existence of trace contaminants such as carbon (C), silicon dioxide (SiO2), iron (III) oxide (Fe2O3), and titanium dioxide (TiO2). The BET surface area and pore volume were determined subsequent to activation at 550 °C for a duration of 3 h, as specified in the brochure provided by Sasol Inorganics. Sasol Inorganics produces alumina and boehmite primarily through synthetic aluminium alkoxide processing routes. Alumina is industrially produced through two methods: co-production with synthetic linear alcohols, also known as the Ziegler method, and direct production from aluminum metal, which is referred to as the on-purpose route. Various production procedures are utilized, which entail the creation of an aqueous intermediate slurry of alumina, in order to achieve additional tailoring. The aforementioned procedures include the precursor substances of alumina hydrates, calcined alumina, and their modified variants. The crystallite size of boehmite is influenced by several experimental conditions during its preparation from the liquid phase. These conditions include the aluminum source, precipitant, solution pH, temperature, aging, and drying conditions, as reported by Okada et al. [20–22].
Characteristics provided by Sasol Inorganics Germany on the chosen grades.
2.2 Characterization techniques and data analysis methodologies
In order to ascertain the existence of organic impurities on the surface of chosen commercial boehmite grades, InfraRed spectroscopy is utilized. The data is collected using a Digilab FTS 3000 computer-driven spectrometer in the transmission mode, within the range of 400–4000 cm−1. The Bragg Brentano geometry was employed to conduct XRD measurements utilizing a BRUKER AXS D8 Discover diffractometer with CuKα1 radiation of 1.54059 Å. The experimental setup included the utilization of a quartz front monochromator a linear detector of the Lynxeye XE-T type with energy resolution, and a motorized anti-scatter screen. The XRD analysis encompassed a scanning range of 2θ values spanning from 10° to 140°, utilizing a step size of 0.02° and a counting duration of 6 s per step.
As for the TEM observations, a suspension of boehmite with a concentration of 0.1 M was prepared in ethanol. In the context of sample preparation, a drop-casting technique was employed to apply a slender coating of the boehmite solution onto a TEM grid, which is comprised of a delicate carbon film that was reinforced on a Cu grid. After drying this grid, 10 nm gold nanoparticles were deposited and dried on it to improve contrast and stability during acquisition. The study utilized a JEOL 2100F microscope in bright field mode, operating at an accelerated voltage of 200 kV with a high tilt holder, to conduct TEM imaging and electron tomography. The alignment process of obtained projections was accomplished utilizing the IMOD software developed by the University of Colorado. The samples were reconstructed using algorithms provided by ImageJ and IMOD software. In order to enhance the fidelity of the reconstructed volumes, targeted filters, such as the median filter, were implemented to eliminate extraneous noise stemming from electron diffraction and interactions with the carbon membrane of the copper grids. Modelling of the segmented volumes was carried out using a 3D Slicer image computing platform. The Fiji software was utilized to observe and pre-process tomographic reconstructions. This involves the application of filters to diminish noise and improve contrast, taking into consideration the signal-to-noise ratio. The process of segmentation involved the separation of materials and pores, generally achieved using semi-manual thresholding based on the bi-modal distribution of the histogram. After the process of segmentation, the morphological and topological parameters are quantified by 3D visualization and modeling. The binary volume differentiates between porous and material phases by representing them as black and white voxels, respectively. The process of noise reduction includes the utilization of erosion and dilation activities. The application of the watershed method facilitated the geometric segregation of interconnected pores, hence assisting in the comprehensive examination of their morphology and offering valuable insights into the hierarchical nature of porosity.
3 Results and discussion
Crystallites are ordered three-dimensional clusters of atoms or molecules. Boehmite is composed of clusters of small aggregates of crystallites due to the hierarchical nature of the material. These aggregates are formed by nanocrystallites joined together by chemical bonds, leading to irreversible aggregation phenomena. However, the agglomeration of these aggregates is a reversible process, involving electrostatic interactions or weak van der Waals bonds. In this study, the smallest clusters of crystallites or particles obtained through constant ultrasonication in solution are referred to as aggregates (the smallest hierarchical unit has been considered as aggregates here as well). In contrast, the clusters obtained without ultrasonication, but simply through dispersion in solution, are referred to as agglomerates.
3.1 Structural analysis by X-ray diffraction and transmission electron microscopy
Figure 1 displays the XRD patterns of the four boehmite samples that were examined. The prominence of the (020) plane among the crystallographic planes is indicated by the intensity of its corresponding peak. The full width at half maximum (FWHM) of the (020) reflection was determined following background subtraction and experimental enlargement.
 |
Fig. 1 X-ray diffraction of different boehmite grades under study after a Rietveld refinement data analysis. |
The XRD patterns obtained for the boehmite grades are very similar to those reported in the literature [23–26]. An anisotropic size model was employed to conduct Rietveld refinement on various boehmite grades, following an examination of both isotropic and anisotropic models.
The structure of boehmite is characterized by the presence of AlO(OH)6 octahedral layers, and its physical properties, including crystallinity and morphology, have been observed to be impacted by the conditions under which it is prepared, as noted by Christoph et al. [27]. According to Tettenhorst and Hofmann’s findings, the (020) crystallographic planes in boehmites synthesized at 300°C can be identified by the peak at 2θ = 14.5°, as observed by Tettenhorst et al. [23, 28]. The XRD analysis results indicate that the average morphology of the crystallites is characterized by crystallites with dimensions that vary between 18 and 25 nm along the [020] direction. The crystallite sizes are measured to be 31 nm along the [002] direction, and between 36 and 41 nm along the [200] direction, as presented in Table 2. It is noteworthy that XRD is utilized to determine the dimensions of the coherent scattering domains present in a crystallite, commonly known as the “crystallite size” or “coherent domain size.” The aforementioned measurement may not consistently correspond to the precise particle size of the material under observation due to crystallite aggregation and resulting polycrystalline grain growth.
Approximate size of the crystallites deduced from the analysis of FWHM of the different diffraction peaks observed in the XRD patterns and average size of platelets obtained from 2D TEM.
3.2 Average size distribution by TEM
TEM imaging techniques are highly suitable for particle size analysis due to their ability to directly measure the size of individual particles. Measurements are conducted manually or automatically along the particle’s longest axis, which can be regarded as perpendicular to the [200] direction, as determined by the morphology derived from XRD. Each boehmite grade was subjected to particle measurement of around 250 particles. The mean particle sizes of TH500, D40, D10F4, and C200 specimens are measured to be 42 ± 12, 42 ± 13, 47 ± 17, and 41 ± 12 nm, respectively. The aforementioned values exhibit notable deviations from the crystallite dimensions derived via XRD analysis, as depicted in Figure 2. Furthermore, it was also observed that particles with a size of up to 180 nm were preferentially aligned parallel to the grid membrane.
 |
Fig. 2 Average particle size distribution of different boehmite grades from TEM (in insert), and typical TEM images of each boehmite under study. |
In a case study of the D10F4 grade, approximately 65% of the particles exhibit dimensions that fall within the size range of 35–55 nm, while a significant proportion of the particles exhibit sizes as small as 20 nm. The size distribution of particles reveals that nearly 5% of the particles exhibit dimensions ranging from 60 to 110 nm, while a small fraction of particles display dimensions approaching 200 nm. The presence of larger crystallites in commercially produced bulk samples is a common occurrence that can be attributed to irregular expansion or growth during the synthesis process. This variation in crystallite sizes is an ordinary outcome of the production process.
The TH500 and D40 grades exhibit a Gaussian size distribution curve that is positively skewed with respect to particle size distribution. Approximately 75% to 80% of particles are found to be within the size range of 35–55 nm. A minor proportion of very small particles is detected, while approximately 3% of particles exhibit larger dimensions within the range of 60–85 nm. A minor proportion (0.3%) of particles measuring around 95 nm in size is also detected. Comparable patterns are observed in the D40 category, featuring the incorporation of particles within the 55–65 nm magnitude, albeit in greater ratios. Merely 1.5% of particles exhibit dimensions that fall within the range of 65–110 nm.
The Boehmite grade C200 displays discernible patterns in contrast to the remaining grades. The observed distribution of particle sizes exhibits a bimodal pattern, wherein particles are equally distributed in the size ranges of 20–40 and 40–55 nm. The variability observed in the distribution of particle sizes is plausibly linked to the particular method of synthesis utilized for the C200 specimen, which entails this grade as high-density alumina hydrate, as posited by Sasol Ltd. The C200 sample exhibits a scarcity of particles with approximately 60 nm size, in contrast to the other grades. However, there is an absence of significant particles measuring around 100 nm in this sample. The cumulative percentage frequency curve, as illustrated in Figure 2, was utilized to illustrate the particle size distribution. The non-conformity of the size distribution to a Gaussian distribution indicates the presence of considerable irregularity in particle shapes, that is, deviation from the regular cubic shape, as well as a broad range of particle size distribution (Fig. 3).
 |
Fig. 3 Structural features extracted from the electron tomographic data. 3D model and typical 2D TEM image extracted from the tilt series of a (a) TH500 agglomerate; (b) D40 agglomerate; (c) D10F4 agglomerate; (d) C200 agglomerate; and (e) two types of aggregates formed as part of different interactions between crystallite surfaces: F1, F2, F3, F4 – Schematic representations of the basal and lateral face interactions between various crystallites. |
3.3 Particle shape characterization in 2D: “irregularity” parameter
Diverse shape factors can be utilized to describe the morphology of particles, with sphericity being a prevalent metric for assessing the degree of compactness of any given shape. The determination of sphericity can be accomplished by means of two distinct methodologies, namely, dimension measurements and volume with surface area measurements, which are commonly referred to as true sphericity. The parameter denoted as irregularity parameter (IP) is a fundamental approach utilized for the computation of particle sphericity, which is based on the analysis of 2D-TEM images. For anisotropic particles that exhibit a degree of regularity in their shape, such as rods or plates, the parameter used to quantify their irregularity is the ratio of the length of their longest direction (major axes) to the length perpendicular to that direction (minor axes) [29].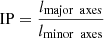
where lmajor axes is the length along the longest axis and lminor axes is the length along the direction perpendicular to the longest axis. When the irregularity parameter approaches 1, it is considered a circle. The average thickness of the platelets was found to be approximately between 15 and 20 nm in size, however, due to the low sampling size regarding the orientation of the platelets, these thicknesses were not taken into account during the measurement of irregularity parameter measurements.
Table 3 presents the results of the calculation of the irregularity parameter (IP) for the different grades of boehmite that were examined, based on the analysis of 2D TEM images. Each grade was subjected to a particle measurement of around 60 particles. The IP values for hexagonal and quadrangular particle shapes have been reported as 1.155 and 1.414, respectively, according to Mikli et al. [30]. The irregularity parameters that were computed for the various grades of boehmite indicate that the particle shape distribution in these grades is probably a composite of hexagonal and quadrangular shapes, rather than a uniform blend of one specific morphology.
Irregularity parameter measured from 2D TEM images for each boehmite grade under study.
3.4 Platelet morphology and 3D shape: a quantitative study
The polyhedral configurations of crystallite are frequently exposed through the utilization of SEM images, which offer clear distinctions among various facets. The recognition of polyhedra is a challenging task when analyzing 2D TEM images, as their projection nature unveils certain difficulties. When the electron beam is aligned with the [001] zone axis, a cubic crystallite can exhibit a square shape in a TEM image. The edges that have been noted are indicative of distinct crystallite facets, namely (100), 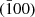 , (010), and
, (010), and 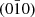 , and their identification can be corroborated through the utilization of SAED patterns. In cases where there are minor variations along the [100] and [010] orientations, the TEM image may exhibit a rectangular morphology. Upon observation of a cubic crystallite along the [110] orientation, a rectangular configuration is noticeable in the TEM picture. The identification of facets in TEM investigations frequently necessitates the manipulation of crystallite orientation in multiple axes or cross-referencing with SEM imagery for comparative purposes. The utilization of lattice fringes and electron diffraction spots in HRTEM images is, therefore, limited to indicating a crystallite orientation that is perpendicular to the electron beam [31, 32].
, and their identification can be corroborated through the utilization of SAED patterns. In cases where there are minor variations along the [100] and [010] orientations, the TEM image may exhibit a rectangular morphology. Upon observation of a cubic crystallite along the [110] orientation, a rectangular configuration is noticeable in the TEM picture. The identification of facets in TEM investigations frequently necessitates the manipulation of crystallite orientation in multiple axes or cross-referencing with SEM imagery for comparative purposes. The utilization of lattice fringes and electron diffraction spots in HRTEM images is, therefore, limited to indicating a crystallite orientation that is perpendicular to the electron beam [31, 32].
Hence, the spatial orientation and morphological variations of the various boehmite grades’ aggregates were analyzed through 3D TEM imaging. The study revealed the presence of four unique particle morphologies, namely elongated hexagonal structures with a longer dimension along the [002] direction, small cubic structures, rhombohedral structures with a shorter dimension along the [020] direction, and regular hexagonal structures (Fig. 4). The determination of the distribution of said morphologies within each grade of boehmite was carried out via an analysis of reconstructed volumes and associated TEM projections. The mean number of particles per aggregate was approximately 15–20, and the particle size exhibited variation based on the morphology. An aggregate refers to a collective structure in which particles are bound together through a combination of van der Waals forces, electrostatic interactions, and chemical bonds. The properties of an aggregate, such as mechanical strength, porosity, or reactivity, exhibit notable distinctions from those of its constituent particles. The elongated hexagonal particles displayed an average size of 151 ± 26 nm, whereas the small cubic, rhombohedral, and regular hexagonal particles had average sizes of 22 ± 4, 41 ± 9, and 55 ± 13 nm, correspondingly. The size distributions of said morphologies exhibited similarity across all four grades of boehmite. Furthermore, electron diffraction investigations were carried out to establish a correlation between the perceived morphologies and particular crystallographic orientations.
 |
Fig. 4 Different morphologies were identified from the different grades; A1 – Elongated hexagonal morphology as deduced, A2 – Size distribution of the elongated hexagonal morphology, B1 – regular hexagonal morphology, B2 – Size distribution of the regular hexagonal morphology, C1 – rhombohedral morphology, C2 – Size distribution of the rhombohedral morphology, D1 – cubic morphology, D2 – Size distribution of the cubic morphology. |
Variable particle morphology can result in size-dependent configurations that have a significant effect on the ratio of crystallographic planes and modify interfacial reactivity. Jolivet et al. [33] have documented the aforementioned occurrence in particles of boehmite (γ-AlOOH) with dimensions spanning from 10 to 100 nm. According to the studies conducted by Jolivet et al. [34], the larger particles, measuring ca. 100 nm, are comprised of fibrous or rod-shaped aggregates that are composed of small platelets. These platelets were found to possess lateral faces of (100) and basal planes of (010). On the contrary, it has been observed that γ-AlOOH nanoparticles ranging from 10 to 25 nm exhibit a distinctive diamond-shaped morphology featuring lateral faces with a [101] orientation. This has been confirmed by the presence of an angle of approximately 104° between the (101) and 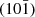 distortions. Modifications in electrostatic surface charge density and surface energy lead to changes in the area ratio between the (100), (010), and (101) faces as the particle size decreases [11]. Similar trends in arrangements have been observed in the morphologies found in our study as well.
distortions. Modifications in electrostatic surface charge density and surface energy lead to changes in the area ratio between the (100), (010), and (101) faces as the particle size decreases [11]. Similar trends in arrangements have been observed in the morphologies found in our study as well.
Quantitative analyses were carried out to determine the frequency of distinct morphologies in every grade of boehmite. Although the grades share certain properties, their distinct morphological distributions set them apart from each other. In Figure 5, the C200 boehmite grade exhibits a noteworthy balanced mixture of morphologies, where regular hexagonal, rhombohedral, and small cubic morphologies are almost equally prevalent in standard aggregates. The C200 aggregate is typically composed of around 26 ± 6 particles, with a minority (4%) exhibiting the fourth type of crystallite morphology.
 |
Fig. 5 Distribution of the four identified morphologies in the aggregates of TH500, C200, D40, and D10F4 boehmite grades. |
The quantification of distinct morphologies present in boehmite aggregates was performed for every grade (Fig. 4). Approximately half of the aggregates in TH500 were composed of rhombohedral structures, whereas D40 displayed a relatively uniform distribution with small cubic structures being the predominant morphology. The average number of crystallites found in TH500 and D40 aggregates was determined to be 27 ± 8 and 53 ± 5, respectively (Fig. 5). The occurrence of elongated hexagonal-shaped structures was infrequent across all grades. Among the aggregates, TH500 exhibited the lowest frequency while D40 exhibited the highest frequency for elongated hexagonal-shaped morphologies. The D40 aggregates exhibited the lowest proportion of rhombohedral and regular hexagonal configurations. The morphological analysis of D10F4 aggregates revealed that the predominant structure type was small cubic, comprising 44% of the total, while rhombohedral structures accounted for approximately 35% of the aggregates. On average, a D10F4 aggregate was composed of approximately 39 ± 7 particles. The quantification of particle count within aggregates was ascertained through the utilization of TEM images and electron tomographic data.
The literature lacks extensive reporting on the coexistence of diverse crystallite morphologies and sizes within aggregates in single synthesis batches of boehmite. Prior research on hydrothermal synthesis pathways has documented the impact of multiple factors, including temperature, duration, concentration, anion species, and pH, on the development of boehmite morphology as explained previously [35–37]. Zhang et al. [7] observed that boehmite nano platelets displayed hexagonal morphology within the pH range of 7–10, whereas they exhibited rhombic morphology in highly alkaline environments (pH = 12–13.3). By manipulating these factors, the nano platelets’ morphology was observed to exhibit a systematic variation between hexagonal and rhombic shapes, thus emphasizing the impact of face-specific effects. Research conducted on the growth morphologies of gibbsite has revealed that there are variations in the process, resulting in truncated lozenge, truncated triangle, and hexagonal platelet shapes [38]. These variations are influenced by various factors, including acid concentration. However, comprehensive, and exhaustive analyses of this phenomenon are outside the scope of this study.
The heterogeneous morphology observed in these boehmite grades can be ascribed to the diverse mechanisms implicated in its industrial chemical production. In the process of chain growth reaction, the resultant aluminum alkyl compound experiences oxidation leading to the formation of aluminum alkoxide. According to Basset et al. [39], the catalytic activity of Lewis acids during hydrocarbon isomerization reactions is promoted by the presence of oxygen, which enhances the electron affinity of Lewis sites. In a study conducted by Fu et al. [40], boehmite crystallization was observed under hydrothermal conditions using water as a mineralizer. The study found that the size of the formed crystallites was positively correlated with higher annealing temperatures. The introduction of water initiates the creation of an intermediary compound known as Al(OH)3, which subsequently undergoes hydrolysis to form γ-AlOOH. This process results in the development of elongated hexagonal shapes in an acidic medium. The presence of a high concentration of H2O has the potential to impede the synthesis of Al(OH)3, thereby promoting the production of colloidal γ-AlOOH. In an acidic environment, the protons present in the reaction solution react with the hydroxyl oxygen-lone pairs, leading to the disruption of the γ-AlOOH layers. The process involves the curling of distinct layers, which subsequently assume elongated hexagonal structures through a scrolling-growth pathway [41]. The rhombic sheet-like morphologies can persist as the pH and water concentration increase since the conditions are insufficient to remove the surface -OH groups, thereby maintaining the γ-AlOOH layers and facilitating the development of the rhombic structures. The present investigation provides evidence in favor of the conjecture regarding the boehmite growth mechanisms through gibbsite and the emergence of diverse morphologies from the fundamental lozenge morphology.
3.5 Particle compactness and sphericity: a quantitative study
Sphericity, a concept coined by Wadell et al. [42, 43] generally refers to the overall form of particles. The term refers to the ratio of the surface area of a sphere with an equivalent volume to the given particle/aggregate and the surface area of the entity itself. Mathematically, sphericity is defined as![$$ \psi =\enspace \frac{\sqrt[3]{36\pi {V}^2}}{\mathrm{SA}} $$](/articles/stet/full_html/2024/01/stet20240016/stet20240016-eq5.gif) where V is the volume of the particle and SA is the surface area of the particle. In this study, particle volume and surface area are measured approximately from the reconstructed surface in units per voxel. Average sphericity measurements were carried out on the aggregates of the four different boehmite grades and were observed that ψ ~ 0.722 for TH500 samples, ψ ~ 0.835 for D40 samples, ψ ~ 0.734 for D10F4 samples and ψ ~ 0.862 for C200 samples. This also suggests the combination of basal and lateral facets’ interaction towards the contribution of the constitution of an aggregate, which in turn represents their compactness during agglomerate formation.
where V is the volume of the particle and SA is the surface area of the particle. In this study, particle volume and surface area are measured approximately from the reconstructed surface in units per voxel. Average sphericity measurements were carried out on the aggregates of the four different boehmite grades and were observed that ψ ~ 0.722 for TH500 samples, ψ ~ 0.835 for D40 samples, ψ ~ 0.734 for D10F4 samples and ψ ~ 0.862 for C200 samples. This also suggests the combination of basal and lateral facets’ interaction towards the contribution of the constitution of an aggregate, which in turn represents their compactness during agglomerate formation.
3.6 Particle contact interfaces
The concluding part of this quantitative study involved the evaluation of the contact surface area among particles that belong to the identical aggregate. A geometrical approach was utilized to ascertain the percentage of the contact surface that arises from the overlap of two crystallographic surface facets that belong to two adjacent particles. Given two overlapping regions on a plane, we defined the bottom left and top right points of the two rectangles (areas I and II of Fig. 6). The total contact area is calculated as the sum of areas I and II. Based on the morphological analysis, it is possible for the planes or regions to exhibit either hexagonal or rectangular geometries. It is noteworthy that the duplicated inclusion of the intersecting region was addressed by deducting its area from the overall calculation of the contact area.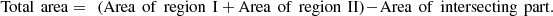
 |
Fig. 6 Methodology adopted for the measurement of the area of the surface contact. |
Insights of significant value can be obtained by analyzing the distribution of morphologies with respect to the contact surface, which sheds light on the interactions among the dominant crystallographic planes, specifically {202}, (020), and (002). Here the crystallographic axis of {202} was assumed based on the angle of approximately 104° between the lateral phases of the truncated lozenge-shaped platelets and similar observations from literature [11] but could not be confirmed using electron diffraction experiments due to the high beam sensitive nature of the samples [44, 45] and the orientation of these faces in different directions in an aggregate [31]. In order to enhance comprehension and visualization, the planes {202}, (002), and (020) have been redefined to {101}, (001), and (010), respectively. The present investigation entailed a thorough examination of the reconstructed volumes, whereby each slice was meticulously scrutinized to quantify the surface contacts of each phase. The quantification and comparison of the contact area for each crystallographic plane were conducted. It is noteworthy that the interaction patterns among the crystallographic planes exhibit distinctiveness for every boehmite grade but these interactions are quantified in terms of rough order of magnitude. The data that has been acquired and is presented in tabular form indicates discernible discrepancies in the distribution of contact surface area across the various boehmite samples. The plane indexed as {101} exhibited the greatest contact surface percentages for D40 (50%), TH500 (44%), and C200 (32%). In contrast, D10F4 demonstrated a comparatively lower percentage of 24%. The results indicate that C200 had the highest contact surface percentage of 78% for the (010) plane, followed by D40 (47%), D10F4 (42%), and TH500 (15%). The (001) crystallographic plane exhibited considerable variability, as evidenced by the varying contact surface percentages of the different materials. TH500 displayed the highest contact surface percentage at 68%, while D10F4, C200, and D40 had contact surface percentages of 35%, 19%, and 21%, respectively. The results suggest that there was a higher level of interactivity between {101} planes in D40 compared to the other grades. In contrast, it was observed that TH500 exhibited a more pronounced interaction between the {101} and {101} planes, as depicted in Figure 7. The analysis of quantitative data related to the contact area between different crystallographic planes contributes to our understanding of the structural properties of particles. The different grades under scope exhibit distinctive nanoscale interactions and distributions, underscoring their inherent differences, despite their similar physical and textural characteristics.
 |
Fig. 7 (a) Assignment of crystallographic planes obtained from literature and electron diffraction experiments, (b) Comparison of the interaction of (001) lateral plane with other major crystallographic planes, (c) Comparison of the interaction of {101} lateral plane with other major crystallographic planes and (d) Comparison of the interaction of (010) basal plane with other major crystallographic planes. |
The data’s graphical depiction provides additional inside regarding the variations in contact surface distribution observed among the boehmite samples. It is also noteworthy that C200 displays a relatively equitable allocation between the {101} and (010) planes, whereas TH500 evinces a greater inclination towards the (001) plane. The sample denoted as D10F4 exhibits a comparatively reduced percentage of contact surface area in comparison to the remaining samples.
The complete comprehension of the specific mechanism responsible for the observed preferential arrangement remains elusive. Our proposal is that the phenomenon in question may involve the formation of hydrogen bonds between surface water molecules and layered water molecules. The presence of hydrogen bonds may have a significant impact on the distinct interactions and configurations that are evident among the crystallographic planes, thereby exerting an influence on the overall morphology and structure of the particles. Additional investigation is necessary to authenticate this hypothesis and acquire a deeper comprehension of the fundamental mechanisms at play.
4 Conclusions
The XRD patterns obtained from a series of different grades of boehmite samples are consistent with the existing literature, thereby validating the prevalence of the (020) plane as the primary crystallographic plane. The use of an anisotropic size model in Rietveld refinement yielded complementary results. Thus, the typical morphology of the crystallites is represented by platelets exhibiting dimensions ranging from 18 to 25 nm along the [020] direction, basal plane extensions measuring 31 nm along [002] direction, and 36–41 nm along [200] direction. For the boehmite grades TH500, D40, D10F4, and C200, particle size measurements made using TEM imaging techniques showed notable differences from the dimensions obtained from XRD analyses. This difference could be attributed to XRD commonly being employed for the purpose of determining the dimensions of a crystallographic coherent domain, as opposed to the overall physical size of the particle or aggregate as discussed previously. The bulk of the particles were between 35 and 55 nm in size, with a size range of 20–180 nm. For the TH500 and D40 grades, the particle size distribution showed a positively skewed Gaussian curve, with the majority of the particles in the 35–55 nm range. A similar distribution was seen in D10F4, however, D10F4 also encompassed bigger particles, between 55 and 65 nm. With particles uniformly dispersed in the 20–40 and 40–55 nm ranges, C200 had a bimodal distribution. The particle shape distribution in the analyzed grades is most likely a combination of hexagonal and quadrangular shapes, according to the irregularity parameter (IP) study of boehmite particles using 2D TEM images.
A variety of morphologies, including extended hexagonal structures, small cubic structures, rhombohedral structures, and hexagonal sheet-like structures, were determined by TEM image analysis. The contact surface area estimation of Boehmite aggregates quantitatively demonstrated unique interactions between the crystallographic planes {101}, (010), and (001). Each grade of boehmite had distinctive contact surface distribution patterns, indicating differences in nanoscale interactions and distributions. In D40, TH500, and C200, the {101} plane displayed the highest contact surface percentages, but D10F4 displayed a significantly lower percentage. The C200 plane showed the greatest variation in the (010) plane’s percentage of contact surface. The contact surface percentage of TH500 showed the largest variance in the (001) plane among the various boehmite grades. While TH500 showed a stronger leaning towards the (001) plane, C200 showed a distribution between the {101} and (010) planes that was more evenly distributed (Table 4).
Comparison of various textural properties of the commercial boehmites under study.
The present study outlines how diverse the microstructure of industrial boehmite may be represented through the size, morphology, and types of surface interactions. These microstructural differences are likely to play an important role in the topology of the porous network by modulating the pore shapes and their interconnections, which are two important aspects governing the molecular transport in such hierarchical porous solids. It is yet unknown what exact mechanism underlies the preferred arrangement of crystallographic planes described. However, it is speculated that the interactions and arrangements between the planes may be shaped by preferential H-bonding at specific crystallographic surfaces modulated by the pH and ionic strength of the synthesis media. To verify this hypothesis and learn more about the underlying systems, additional research is required.
Acknowledgments
The authors would like to thank Dr. Nathalie Schildknecht, the Director of the CARMEN Joint laboratory, for her persistent and unyielding assistance throughout the completion of this work. The Sasol company is acknowledged for providing the boehmite powder samples.
Funding
This work is being carried out as part of the collaborative project called Laboratoire Commun de Recherche de Caractérisation des Matériaux pour les énergies Nouvelles – LCR CARMEN (Characterization of Materials for new Energy). The project involves the participation of IFPEN, CNRS, the University of Strasbourg, and Sorbonne University, Paris. IFP Energies Nouvelles provides the funding for this project as part of NS’s doctoral thesis. The authors would like to thank the DRX and MET platforms of IPCMS, as well as R05 – Direction Physique et Analyse of IFP Energies Nouvelles, for providing the necessary research resources for the effective completion of this study.
References
- Diblitz K., Feldbaum T., Ludemann T. (1998) Manufacturing of raw materials for the catalyst industry, In: Recent Advances In Basic and Applied Aspects of Industrial Catalysis, Proceedings of 13th National Symposium and Silver Jubilee Symposium of Catalysis of India, Elsevier, pp. 599–611. https://doi.org/10.1016/S0167-2991(98)80336-4. [Google Scholar]
- Kaya C., He J.Y., Gu X., Butler E.G. (2002) Nanostructured ceramic powders by hydrothermal synthesis and their applications, Microporous Mesoporous Mater. 54, 37–49. [CrossRef] [Google Scholar]
- Nørskov J.K., Bligaard T., Hvolbæk B., Abild-Pedersen F., Chorkendorff I., Christensen C.H. (2008) The nature of the active site in heterogeneous metal catalysis, Chem. Soc. Rev. 37, 2163–2171. [CrossRef] [PubMed] [Google Scholar]
- Mavrič A., Valant M., Cui C., Wang Z.M. (2019) Advanced applications of amorphous alumina: From nano to bulk, J. Non-Cryst. Solids 521, 119493. [CrossRef] [Google Scholar]
- Brühne S., Gottlieb S., Assmus W., Alig E., Schmidt M.U. (2008) Atomic structure analysis of nanocrystalline boehmite AlO(OH), Cryst. Growth Des. 8, 489–493. [CrossRef] [Google Scholar]
- Karger-Kocsis J., Lendvai L. (2018) Polymer/boehmite nanocomposites: a review, J. Appl. Polym. Sci. 135, 45573. [CrossRef] [Google Scholar]
- Zhang X., Cui W., Page K.L., Pearce C.I., Bowden M.E., Graham T.R., Shen Z., Li P., Wang Z., Kerisit S., N’Diaye A.T. (2018) Size and morphology controlled synthesis of boehmite nanoplates and crystal growth mechanisms, Cryst. Growth Des. 18, 3596–3606. [CrossRef] [Google Scholar]
- Panias D., Asimidis P., Paspaliaris I. (2001) Solubility of boehmite in concentrated sodium hydroxide solutions: model development and assessment, Hydrometallurgy 59, 15–29. [CrossRef] [Google Scholar]
- Jiao W., Wu X., Xue T., Li G., Wang W., Wang Y., Wang Y.M., Tang Y., He M.Y. (2016) Morphological controlled growth of nanosized boehmite with enhanced aspect ratios in an organic additive-free cationic–anionic double hydrolysis method, Cryst. Growth Des. 16, 5166–5173. [CrossRef] [Google Scholar]
- Petrakli F., Arkas M., Tsetsekou A. (2018) α-Alumina nanospheres from nano-dispersed boehmite synthesized by a wet chemical route, J. Amer. Ceram. Soc. 101, 3508–3519. [CrossRef] [Google Scholar]
- Jolivet J.P., Froidefond C., Pottier A., Chanéac C., Cassaignon S., Tronc E., Euzen P. (2004) Size tailoring of oxide nanoparticles by precipitation in aqueous medium. A semi-quantitative modelling, J. Mater. Chem. 14, 3281–3288. [CrossRef] [Google Scholar]
- Adschiri T., Hakuta Y., Sue K., Arai K. (2001) Hydrothermal synthesis of metal oxide nanoparticles at supercritical conditions, J. Nanoparticle Res. 3, 227–235. [CrossRef] [Google Scholar]
- Chen X.Y., Huh H.S., Lee S.W. (2007) Hydrothermal synthesis of boehmite (γ-AlOOH) nanoplatelets and nanowires: pH-controlled morphologies, Nanotechnology 18, 285608. [CrossRef] [Google Scholar]
- Pardo P., Montoya N., Alarcón J. (2015) Tuning the size and shape of nano-boehmites by a free-additive hydrothermal method, Cryst. Eng. Comm. 17, 2091–2100. [CrossRef] [Google Scholar]
- He T., Xiang L., Zhu S. (2009) Different nanostructures of boehmite fabricated by hydrothermal process: effects of pH and anions, Cryst. Eng. Comm. 11, 1338–1342. [CrossRef] [Google Scholar]
- Chiche D., Chanéac C., Revel R., Jolivet J.-P. (2006) Size and shape control of γ-AlOOH boehmite nanoparticles, a precursor of γ-Al2O3 catalyst, Stud. Surf. Sci. Catal. 162, 393–400. [CrossRef] [Google Scholar]
- Zhang L., Jiao X., Chen D., Jiao M. (2011) γ‐AlOOH nanomaterials with regular shapes: hydrothermal fabrication and Cr2O7 2− adsorption, Eur. J. Inorg. Chem. 2011, 5258–5264. [CrossRef] [Google Scholar]
- Kim K.S., Kingston C.T., Hrdina A., Jakubinek M.B., Guan J., Plunkett M., Simardl B. (2014) Hydrogen-catalyzed, pilot-scale production of small-diameter boron nitride nanotubes and their macroscopic assemblies, ACS Nano 8, 6211–6220. [CrossRef] [PubMed] [Google Scholar]
- Otte A., Park K. (2022) Transitioning from a lab-scale PLGA microparticle formulation to pilot-scale manufacturing, J. Control. Release 348, 841–848. [CrossRef] [Google Scholar]
- Okada K., Hattori A., Taniguchi T., Nukui A., Das R.N. (2000) Effect of divalent cation additives on the γ‐Al2O3‐to‐α‐Al2O3 phase transition, J. Am. Ceram. Soc. 83, 928–932. [CrossRef] [Google Scholar]
- Okada K., Hattori A., Kameshima Y., Yasumori A., Das R.N. (2000) Effect of monovalent cation additives on the γ‐Al2O3‐to‐α‐Al2O3 phase transition, J. Am. Ceram. Soc. 83, 1233–1236. [CrossRef] [Google Scholar]
- Okada K., Nagashima T., Kameshima Y., Yasumori A., Tsukada T. (2002) Relationship between formation conditions, properties, and crystallite size of boehmite, J. Colloid Interface Sci. 253, 308–314. [CrossRef] [Google Scholar]
- Tettenhorst R. (1980) Crystal chemistry of boehmite, Clays Clay Miner. 28, 373–380. [CrossRef] [Google Scholar]
- Kloprogge J.T., Duong L.V., Wood B.J., Frost R.L. (2006) XPS study of the major minerals in bauxite: gibbsite, bayerite and (pseudo-)boehmite, J Colloid Interface Sci. 296, 572–576. [CrossRef] [Google Scholar]
- Wang Y.G., Bronsveld P.M., DeHosson J.T., Djuričić B., McGarry D., Pickering S. (1998) Ordering of octahedral vacancies in transition aluminas, J. Am. Ceram. Soc. 81, 1655–1660. [CrossRef] [Google Scholar]
- Boumaza A., Favaro L., Lédion J., Sattonnay G., Brubach J.B., Berthet P., Huntz A.M., Roy P., Tétot R. (2009) Transition alumina phases induced by heat treatment of boehmite: an X-ray diffraction and infrared spectroscopy study, J Solid State Chem. 182, 1171–1176. [CrossRef] [Google Scholar]
- Christoph G.G. (1979) The crystal structure of boehmite, Clays Clay Miner. 27, 81–86. [CrossRef] [Google Scholar]
- Tettenhorst R.T., Corbató C.E. (1988) Comparison of experimental and calculated X-ray powder diffraction data for boehmite, Clays Clay Miner. 36, 181–183. [CrossRef] [Google Scholar]
- Ulusoy U. (2023) A review of particle shape effects on material properties for various engineering applications: from macro to nanoscale, Minerals 13, 91. [CrossRef] [Google Scholar]
- Mikli V., Käerdi H., Kulu P., Besterci M. (2001) Characterization of powder particle morphology, Proc. Estonian Acad. Sci. Eng. 7, 22–34. [CrossRef] [Google Scholar]
- Zhou W., Greer H.F. (2016) What can electron microscopy tell us beyond crystal structures?, Eur. J. Inorg. Chem. 2016, 941–950. [CrossRef] [Google Scholar]
- Florea I., Feral-Martin C., Majimel J., Ihiawakrim D., Hirlimann C., Ersen O. (2013) Three-dimensional tomographic analyses of CeO2 nanoparticles, Cryst. Growth Des. 13, 1110–1121. [CrossRef] [Google Scholar]
- Chiche D., Digne M., Revel R., Chanéac C., Jolivet J.-P. (2008) Accurate determination of oxide nanoparticle size and shape based on X-ray powder pattern simulation: application to boehmite AlOOH, J. Phys. Chem. 112, 8524–8533. [Google Scholar]
- Chiche D., Chizallet C., Durupthy O., Chanéac C., Revel R., Raybaud P., Jolivet J.P. (2009) Growth of boehmite particles in the presence of xylitol: morphology oriented by the nest effect of hydrogen bonding, Phys. Chem. Chem. Phys. 11, 11310–11323. [CrossRef] [PubMed] [Google Scholar]
- Bokhimi X., Sánchez-Valente J., Pedraza F. (2002) Crystallization of sol-gel boehmite via hydrothermal annealing, J. Solid State Chem. 166, 182–190. [CrossRef] [Google Scholar]
- Mishra D., Anand S., Panda R.K., Das R.P. (2000) Hydrothermal preparation and characterization of boehmites, Mater. Lett. 42, 38–45. [CrossRef] [Google Scholar]
- Liu Y., Ma D., Han X., Bao X., Frandsen W., Wang D., Su D. (2008) Hydrothermal synthesis of microscale boehmite and gamma nanoleaves alumina, Mater. Lett. 62, 1297–1301. [CrossRef] [Google Scholar]
- Louaer S., Wang Y., Guo L. (2013) Fast synthesis and size control of gibbsite nanoplatelets, their pseudomorphic dehydroxylation, and efficient dye adsorption, ACS Appl. Mater. Interfaces 5, 9648–9655. [CrossRef] [PubMed] [Google Scholar]
- Basset J.M., Taarit Y.B., Coudurier G., Praliaud H. (1974) The effect of oxygen on Lewis acidity of aluminum alkyl compounds and its promoting effect on cocatalysts in metathesis, J. Organomet. Chem. 74, 167–173. [CrossRef] [Google Scholar]
- Fu G.F., Wang J., Xu B., Gao H., Xu X.-L., Cheng H. (2010) Influence of hydrothermal temperature on structure and microstructure of boehmite, Trans. Nonferrous Met. Soc. China 20, s221–s225. [CrossRef] [Google Scholar]
- Chen X.Y., Lee S.W. (2007) pH-Dependent formation of boehmite (γ-AlOOH) nanorods and nanoflakes, Chem. Phys. Lett. 438, 279–284. [CrossRef] [Google Scholar]
- Zhao B., Wang J. (2016) 3D quantitative shape analysis on form, roundness, and compactness with μCT, Powder Technol. 291, 262–275. [CrossRef] [Google Scholar]
- Wadell H. (1932) Volume, shape, and roundness of rock particles, J. Geol. 40, 443–451. [CrossRef] [Google Scholar]
- Jiang N. (2016) Electron beam damage in oxides: a review, Rep. Prog. Phys. 79, 016501. [CrossRef] [MathSciNet] [PubMed] [Google Scholar]
- Arami H., Mazloumi M., Khalifehzadeh R., Sadrnezhaad S.K. (2007) Electron beam-induced “nanocalcination” of boehmite nanostrips to mesoporous α-alumina phase, J. Am. Ceram. Soc. 90, 3311–3313. [CrossRef] [Google Scholar]
All Tables
Approximate size of the crystallites deduced from the analysis of FWHM of the different diffraction peaks observed in the XRD patterns and average size of platelets obtained from 2D TEM.
Irregularity parameter measured from 2D TEM images for each boehmite grade under study.
Comparison of various textural properties of the commercial boehmites under study.
All Figures
 |
Fig. 1 X-ray diffraction of different boehmite grades under study after a Rietveld refinement data analysis. |
| In the text | |
 |
Fig. 2 Average particle size distribution of different boehmite grades from TEM (in insert), and typical TEM images of each boehmite under study. |
| In the text | |
 |
Fig. 3 Structural features extracted from the electron tomographic data. 3D model and typical 2D TEM image extracted from the tilt series of a (a) TH500 agglomerate; (b) D40 agglomerate; (c) D10F4 agglomerate; (d) C200 agglomerate; and (e) two types of aggregates formed as part of different interactions between crystallite surfaces: F1, F2, F3, F4 – Schematic representations of the basal and lateral face interactions between various crystallites. |
| In the text | |
 |
Fig. 4 Different morphologies were identified from the different grades; A1 – Elongated hexagonal morphology as deduced, A2 – Size distribution of the elongated hexagonal morphology, B1 – regular hexagonal morphology, B2 – Size distribution of the regular hexagonal morphology, C1 – rhombohedral morphology, C2 – Size distribution of the rhombohedral morphology, D1 – cubic morphology, D2 – Size distribution of the cubic morphology. |
| In the text | |
 |
Fig. 5 Distribution of the four identified morphologies in the aggregates of TH500, C200, D40, and D10F4 boehmite grades. |
| In the text | |
 |
Fig. 6 Methodology adopted for the measurement of the area of the surface contact. |
| In the text | |
 |
Fig. 7 (a) Assignment of crystallographic planes obtained from literature and electron diffraction experiments, (b) Comparison of the interaction of (001) lateral plane with other major crystallographic planes, (c) Comparison of the interaction of {101} lateral plane with other major crystallographic planes and (d) Comparison of the interaction of (010) basal plane with other major crystallographic planes. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.




