| Issue |
Sci. Tech. Energ. Transition
Volume 79, 2024
Decarbonizing Energy Systems: Smart Grid and Renewable Technologies
|
|
|---|---|---|
| Article Number | 91 | |
| Number of page(s) | 12 | |
| DOI | https://doi.org/10.2516/stet/2024072 | |
| Published online | 05 November 2024 | |
Revew Article
Revolutionizing bioenergy: the microalgae-microbial fuel cell frontier
Faculty of Chemical and Process Engineering Technology, Universiti Malaysia Pahang Al-Sultan Abdullah, Lebuhraya Tun Razak, 26300 Gambang, Pahang, Malaysia
* Corresponding authors: adulla672002@gmail.com (A.M. Osman); mazlina@ump.edu.my (A. Noormazlinah)
Received:
12
December
2023
Accepted:
21
August
2024
Microalgae-Microbial Fuel Cell (M-MFC) technology stands out as a highly promising innovation at the nexus of renewable energy and environmental conservation. This cutting-edge approach utilizes microorganisms, including bacteria and algae, to convert the chemical energy in wastewater into electricity, addressing both wastewater treatment and electricity generation. M-MFC relies on microorganisms to convert chemical energy, utilizing components readily available in wastewater, making it a sustainable energy source with considerable potential. Beyond its eco-friendly electricity generation, M-MFC offers cost-effective electricity production, alleviating expenses associated with wastewater treatment and overall electricity consumption. In this comprehensive review, we explore the intricate bio-electrochemical mechanism of M-MFC, shedding light on recent developments and applications. The discussion encompasses crucial factors influencing M-MFC performance, and its essential elements and functions. This review examines the MFC system, particularly M-MFCs, with a focus attention to the functions of key elements such as the anode, cathode, and microorganisms. Additionally, it delves into the material design and configuration of M-MFCs. Furthermore, the review addresses current issues and limitations related to M-MFC systems.
Key words: Microalgae-Microbial Fuel Cell (M-MFC) Biotechnology / Bioelectricity; Biomass; Lipid; Biodiesel / Wastewater treatment / CO2 sequestration
© The Author(s), published by EDP Sciences, 2024
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abbreviations
M-MFC: Microalgae assisted Microbial Fuel Cell
1 Introduction
Currently, human civilization is grappling with a dual challenge of diminishing energy resources and environmental degradation. This critical situation stems from the rapid depletion of fossil fuels and escalating concerns about global climate change, both of which are directly linked to the excessive use of conventional fuels. Industrialization and population growth have further accelerated the consumption of fossil fuels, leading to severe environmental pollution, threats to biodiversity, increased CO2 emissions, and exacerbation of global warming. This has resulted in adverse effects such as floods, wildfires, hurricanes, and disruptions to ecosystems. The energy crisis is worsened by the rising global energy demand and the diminishing reserves of fossil fuels. As a result, researchers and scientists are intensifying efforts to find sustainable and economically feasible renewable energy sources to address these challenges [1, 2].
Significantly, there has been a notable increase in carbon dioxide concentration due to fossil fuel combustion, rising from 388.5 ppm in 2009 to 409.95 ppm in 2019, marking a concerning surge within just ten years [3]. Global society continues to heavily depend on fossil fuels for energy provision and electricity generation [4]. Therefore, it is crucial to explore alternative clean energy sources to ensure energy security and reduce carbon dioxide (CO2) emissions. Various clean energy technologies, such as solar, hydro, wind, biomass, tidal, geothermal, and wave power, have been rapidly advancing. However, a key challenge associated with these renewable resources is efficiently storing and transporting the energy they generate, despite their environmental advantages over fossil fuels [5].
Photosynthesis involves the conversion of solar energy into chemical energy, with algae demonstrating the highest conversion efficiencies among photosynthetic organisms, reaching levels of up to 9% [6]. Microalgae offers numerous advantages over conventional photosynthetic plants, including rapid growth, lack of resource competition, and adaptability to non-arable land. While typical photosynthetic plants achieve energy conversion efficiencies ranging from 4.6% to 6%, microalgae have displayed remarkable efficiency of up to 9% [7]. Additionally, microalgae excel in capturing CO2 and removing nutrients from wastewater, while also holding promise for bioengineering applications [8]. The process of photosynthesis begins with the absorption of light photons and concludes with the conversion of carbon into various compounds like carbohydrates, lipids, and proteins, along with the release of oxygen. Consequently, the utilization of living algae or algal biomass for energy production presents a viable strategy.
Recent progress has brought attention to electrochemical energy storage devices like micro-supercapacitors, supercapacitors, two-dimensional materials, and bioelectricity devices for their high-power density, quick charge/discharge rates, and extended lifespans [9, 10]. Bioelectricity devices, in particular, offer a potentially sustainable solution by generating electricity from organic matter through various biological processes, thus simultaneously addressing wastewater treatment and CO2 sequestration [11]. Recent research has explored leveraging photosynthesis to produce bioelectricity, hydrogen, and other biofuels as alternatives to fossil fuels [3].
Microalgae possess qualities that make them well-suited for use in MFCs and as potential biomass sources. The combination of microalgae with MFCs has attracted attention because microalgae can serve as both oxygen generators and electron acceptors in the cathode chamber [12].
The integration of microalgae with MFCs shows great potential in tackling energy shortages and maintaining water quality [13]. Microalgae-assisted MFCs are considered effective solutions for generating electricity, removing pollutants, and simultaneously treating wastewater, as demonstrated in Table 1. This table illustrates the capabilities of microalgae-assisted MFCs in addressing challenges related to energy and water quality, providing solutions for power generation, pollutant removal, and wastewater treatment.
Scientists have been intrigued by the potential of microalgae to aid MFCs due to their enhanced ability to generate electricity, treat wastewater efficiently, and produce biofuels from microalgal biomass (see Table 1) [14].
The findings presented in Table 1 indicate significant promise for microalgal-assisted MFCs in both wastewater treatment and electricity production. The noteworthy removal of Chemical Oxygen Demand (COD), substantial reduction in nutrients, and high-power densities are encouraging outcomes. Nevertheless, it’s important to acknowledge variations in results and assess the practical scalability and cost-effectiveness for real-world applications.
The latest developments in MFCs have greatly expanded the potential for generating bioelectricity through microbial metabolism. Electroactive microorganisms like bacteria and yeast participate in biocatalytic reactions within MFCs to generate pure bioelectricity [15]. Although bacteria are commonly used in MFCs, many are inefficient at producing electrical current and may require significant feeding and efficient electron acceptors, which can be expensive [6]. In this regard, highly bioactive microalgae that produce oxygen present a promising alternative to bacteria-assisted MFCs. On the cathodic side of MFCs, oxygen acts as a continuous electron acceptor, while photosynthesis supplies energy for current generation on the anode side.
Electrochemical microalgae have shown more favorable outcomes in terms of electricity generation and energy consumption compared to bacteria [15].
Recent advances in MFCs have brought forth innovative opportunities for integrating microalgae, enabling the use of algal biomass for electricity generation [16]. While most laboratory-scale photobioreactors typically rely on artificial lighting, such as fluorescent lamps, to meet the light requirements of microalgae, this practice escalates operational costs and perpetuates reliance on fossil fuels. However, this constraint may be overcome by tapping into the bioenergy potential of microalgae in alternative applications [17].
In recent years, numerous studies have investigated the use of microalgal-assisted MFCs for both wastewater treatment and simultaneous electricity generation. Drawing on data collected over the past five years, this research offers a comprehensive overview of the current status of microalgal-assisted MFCs. Previous reviews have predominantly focused on system configurations, including single-chambered and double-chambered setups, as well as Microbial Electrolysis Cells (MEC) [14].
This review examines the MFC system, focusing on both traditional MFCs and M-MFCs, with particular attention to the functions of key elements such as the anode, cathode, and microorganisms. Additionally, it delves into the material design and configuration of M-MFCs. Furthermore, the review addresses current issues and limitations related to M-MFC systems.
2 Conventional microbial fuel cell (MFC) system
Microbial fuel cell technology is a cutting-edge method for wastewater treatment that involves the use of microorganisms to generate electricity by oxidizing organic matter present in the wastewater. It converts the chemical energy of a fuel (wastewater/organic substrate) into electrical energy with the aid of biocatalytic reactions carried out by microorganisms [18]. Thus, MFCs provide new opportunities for the sustainable production of energy, in the form of electricity produced directly from biodegradable compounds. This technology has several advantages over conventional wastewater treatment methods, such as being more energy-efficient, sustainable, and cost-effective.
Principally, the process of microbial fuel cell technology [19] involves the use of an anode and a cathode separated by a Proton Exchange Membrane (PEM). The anode is placed in the wastewater in anaerobic condition and the cathode is exposed to air. The microorganisms present in the wastewater oxidize organic matter and produce carbon dioxide, protons, and electrons. These electrons are transferred to the anode and electrons pass through an external circuit, generating an electrical current. Protons cross the membrane to the cathode. In the cathode compartment, protons and electrons are combined with an electron acceptor, usually oxygen, to produce water [20–22].
Within the MFC, a PEM separates the two compartments (see Fig. 1) [23]. The cathode is equipped with catalysts to enhance its efficiency, while the anode contains either wastewater or a medium enriched with organic substances. Bacterial colonies are typically cultivated under anaerobic conditions, utilizing acetate, glucose, or wastewater as their primary substrates, and adhering to the negatively charged anode electrode. The bacteria’s metabolism leads to the breakdown of organic matter, releasing energy in the form of protons and electrons, which are transferred to the anode electrode (via a PEM, or proton exchange membrane). The electrons then travel through the external circuit, ultimately reaching the cathode electrode [24].
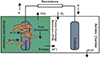 |
Fig. 1 Schematic of a typically employed two-chamber microbial fuel cell highlighting the various electrochemical and electro-microbiological processes. Figure adapted from reference [23]. |
Within the cathode chamber, the electron combines with oxygen and a proton, leading to the formation of water, as illustrated in Figure 1. The figure depicts a PEM positioned in the center of a schematic diagram of a typical MFC, comprising anode and cathode chambers. Microbial Fuel Cells (MFCs) offer dual advantages by generating sustainable electricity and providing an eco-friendly solution for wastewater treatment. Through the utilization of microorganisms, MFCs convert organic matter into clean, renewable electricity while concurrently breaking down pollutants in wastewater. This not only mitigates the environmental impact of wastewater discharge but also reduces operating and maintenance expenses, rendering MFCs a cost-effective and scalable technology for various applications [25, 26].
Notwithstanding their benefits, MFCs come with limitations. They often produce low power densities, which restricts their suitability for high-demand electricity applications. As noted by Shuiliang and colleagues in 2018 [27], despite numerous advancements, the widespread adoption of Microbial Fuel Cell (MFC) technology for power generation still faces significant challenges.
One major challenge is the relatively modest power output observed in larger systems. For instance, a 90-L reactor with a cathode-specific area of 6 m2 m−3 achieves only about 1 W m−3. This limitation hinders the scalability of MFCs for large-scale Wastewater Treatment (WWT), necessitating the use of a greater number of reactors with smaller individual volumes. Treatment rates can be slow, especially with larger wastewater volumes, resulting in prolonged retention times. MFC performance is also sensitive to environmental conditions, requiring careful maintenance of microbial communities. Commercial adoption and standardization practices are still evolving, presenting challenges for widespread implementation. Factors such as the physical space required, initial investment, and technical expertise can serve as barriers, particularly in constrained environments. Researchers are working to address these limitations to enhance MFC effectiveness in sustainable energy and wastewater treatment [28].
3 The developing integration of microalgae in MFCs
Microalgal-assisted Microbial Fuel Cells (MFCs) are an advanced technology in the field of Bioelectrochemical Systems (BES) [29]. This advancement utilizes microalgae to serve as an oxygen source, thereby reducing operational expenses by eliminating the need for external aeration. Cultivating microalgae in MFCs typically involves two methods: introducing live microalgae or using deceased biomass as a substrate. When live microalgae inhabit the cathode, they provide oxygen, allowing it to function as an electron acceptor [30]. Microbial fuel cells (MFCs), without the assistance of microalgae, are technologies capable of both generating electricity and remediating wastewater. Microorganisms convert the energy derived from organic matter present in wastewater into electricity. The cathode receives the protons and electrons generated at the electrode as a result of microbial metabolism. Protons pass through a separator, often a PEM, while electrons travel through an external circuit. Platinum is typically used at the cathode to catalyze the reduction of ambient oxygen, resulting in the formation of water when combined with protons. In bioelectrochemical systems, the substrate is a crucial component, and its type and concentration are important factors affecting the composition of microbes and, consequently, power output [31].
The use of simple organic compounds such as acetate is common because of their high-power output and ease of manipulation [32]. To generate a significant amount of electricity, however, we need to investigate feed-stock options that are not only significantly more affordable but also widely available. In this particular setting, the biomass of microalgae can be put to use. The streams are polluted as a result of microalgae. They are the most important contributors to eutrophication. When wastewater is treated around the world, a significant amount of microalgae biomass is collected as a byproduct. Human health may be in danger if microalgae are discharged directly into the sewer system. Thus, microalgae must be removed from water bodies and thrown away. Biomass from microalgae is used to make biofuels. However, it is not currently economically feasible to produce biofuels from the biomass of microalgae. Microalgae can be utilized as a substrate alternatively in MFC. This strategy combines the production of electricity and trash treatment. Therefore, using microalgae has two advantages: it reduces pollution and serves as a fuel for MFC. Microalgae biomass has a high concentration of proteins (32%) and carbohydrates (51%), both of which can be easily broken down by electro-gens to produce electricity. Daud et al. [3] used Chlorella vulgaris (a microalga) powder as a substrate and attained the maximal power density at 0.98 W/m2 (277 W/m3).
In the past, expensive catalysts (such as Pt or CuO) have been utilized to improve the cathode’s performance. A suitable level of dissolved oxygen can be added to water and circulated as an alternative. The amount of dissolved oxygen present should be equal to the amount of oxygen created by the catalysts. Nevertheless, the ongoing pumping of water increases the operating costs of the MFC. A possible strategy to boost cathodic performance involves substituting catalysts for photosynthetic microalgae species. As microalgae develop, oxygen is released, acting as a terminal electron acceptor. Carbon fixation is a benefit of employing microalgae as a cathode. Then, CO2 is produced by the electrogens during MFC operation. At the cathode, microalgae use CO2 as a source of carbon and encourage growth. Biofuels (biodiesel, bio-hydrogen, and bioethanol) can be extracted from biotechnological, which is a substance produced in large quantities by the cultivation of microalgae [34].
M-MFCs have the following benefits over other bioenergy production technologies: (a) Immediate energy produced out of a substrate, (b) efficiency at room temperature, (c) no need for an external energy source, (d) dependable baseload power, (e) affordable feedstock storage, and (f) low environmental impact are all characteristics of this technology. However, it has some limited drawbacks such as using cost expensive materials such as platinum (Pt) in at cathode chamber [35]. To produce a substantial amount of hydrogen through Microbial Electrolysis Cell (MEC), it is required efficient anode electrolyte is required to produce high current density production and high optimum columbic efficiency. Anode performance is still not sufficient to enable commercial consideration of this system. A power density of 1 kW/m3 has been proposed as a target sufficient to support application development. This is because utilization of microalgae in MFCs is among the most promising approaches. Among other aspects, microalgae can be utilized as a substrate at the anode to extract nutrients or to capture the CO2 produced in the cathode.
There are a variety of benefits to using photo-bioreactors, which have been described in the literature. They grow a lot of biomass, are excellent for outdoor cultivation, and have a large surface area exposed to light. Additionally, they ease control and lessen the possibility of contamination. Photo-bioreactors, however, have a number of drawbacks, including the high costs of operation and the output capital needed. They are intricate systems that require protection against oxygen buildup, biofouling, and shear stress-induced cell damage. Furthermore, the type of bioreactor chosen relies on the microalgae strain, the location, the size of the space provided, and the type of the desired end product [36].
4 The roles of key components in MFCs and M-MFCs
A typical MFC designed for power generation comprises both an anode chamber and a cathode chamber, with a PEM positioned between them. Each chamber is equipped with two distinct types of electrodes, specifically the cathode and the anode. Various components of the M-MFC are illustrated in Figure 2.
 |
Fig. 2 Microalgae-Microbial Fuel Cell (M-MFC). |
4.1 The role of the anode
In a traditional MFC, the anaerobic anode chamber is constructed to receive organic compounds or substrates that undergo microbial oxidation, typically sourced from various organic wastes or wastewater. The main goal is to facilitate the microbial metabolism of these organic compounds, leading to the production of electrons by exoelectrogenic microorganisms. The anode chamber serves a crucial role in supporting the growth and activity of microorganisms capable of transferring electrons to the anode electrode, forming the basis of the electrochemical process. The electrons generated during microbial metabolism are then channelled through an external circuit from the anode to the cathode, where they take part in the reduction reaction. In the MFC system, microorganisms residing on the anode produce electrons through the consumption of organic matter. These electrons subsequently travel to the anode via self-generated mediators or nanowires [37].
In contrast, the anode chamber of a Microalgae-assisted Microbial Fuel Cell (M-MFC) introduces a unique approach. In this system, dead microalgae biomass serves as a substrate in the anode chamber. The microbial oxidation of this dead microalgae biomass becomes the primary mechanism for electron generation. This distinctive feature allows for a dual source of electrons within the anode chamber, with both organic substrates and dead microalgae contributing to the overall electron flow.
In 2018, Ndayisenga and his colleagues conducted a study focusing on the efficiencies and mechanisms of utilizing microalgal biomass for anaerobic respiration in a double-chamber MFC. They chose Chlorella regularis as the model microalgae due to its widespread distribution in aquifers. Initially, they investigated the components of C. regularis to determine its suitability as an anolyte. Subsequently, they examined the electrochemical characteristics of the MFC with C. regularis serving as the sole electron donor [38].
Furthermore, the M-MFC system extends its functionality beyond electricity generation. While dead microalgae are utilized for electron production in the anode chamber, the cathode chamber incorporates live and fresh microalgae. This integration introduces a dual-functionality where microalgae contribute not only to the generation of oxygen in the cathode but also serve as a substrate in the anode. This innovative coupling of dead and live microalgae in different chambers of the MFC system adds complexity and versatility to its capabilities.
The major differences between a typical MFC anode chamber and an M-MFC anode chamber lie in the substrate used, the dual source of electrons, and the additional functionalities, such as wastewater treatment and lipid production, embedded in the innovative M-MFC system. This approach showcases the integration of both dead and live microalgae for a comprehensive and sustainable Microbial Fuel Cell system.
Exoelectrogens, predominantly bacteria, play a pivotal role in generating electrical energy by oxidizing organic substances and transferring the resulting electrons to an external electron acceptor. The movement of electrons from the anode chamber to the cathode is facilitated by an external circuit. Exoelectrogens are also responsible for proton production, and the transfer of protons from the anode to the cathode through the PEM depends on charge mobility and differential charge [3].
Enhancing the microbial electron transfer rate at the anode can be achieved through various approaches, including optimizing cell design, electrode materials, and the introduction of electron mediators. The anode material must possess specific characteristics to support the formation of an active biofilm. A biofilm is an Extracellular Polymeric Substance (EPS), typically enclosed in a self-produced polymeric matrix primarily composed of polysaccharides. This term is commonly used to describe a surface-attached microbial community. As the anode surface provides an ideal environment for respiration, the microbial community forms a biofilm with a thickness of over 30 mm. While the underlying cells are limited in their access to Carbon/Electron (C/E) substrate, the conductive properties of the biofilm matrix allow electrons to efficiently reach the anode. This conductive biofilm matrix becomes an integral part of the anode and is often referred to as the biofilm anode.
When exoelectrogens are employed in a continuous MFC process, carbon materials like cloth, fibers, or veils become an excellent choice for the anode due to their porous characteristics. This type of anode substrate allows for the efficient distribution of the substrate throughout the entire cell through advective transport. However, the presence of non-permeable electrodes, such as rods or graphite plates used in biofilm formation, can result in a thinner cell structure. This, in turn, leads to a reduction in power generation and a lower metabolic rate [39]. A polymer material like polyaniline or Polytetrafluoroethylene (PTFE) with substantial conductivity can also serve as a favored anode electrode. Research conducted by Qiao and colleagues demonstrated that incorporating carbon nanotubes into the electrode structure in MFC could enhance both electron transfer feasibility and increase the electrode surface area.
4.2 The role of the cathode
The electrons from the anode chamber are transferred through an external circuit to the cathode, allowing reduction reactions to take place, usually facilitated by a cathodic catalyst. In certain MFC setups, such as those with a two-chamber design, a membrane (such as a cationic, anionic, or ultrafiltration membrane) is positioned between the anode and cathode to prevent electrical short-circuiting and reduce oxygen infiltration to the Anode-Respiring Bacteria (ARB) [37].
The M-MFC system extends its functionality beyond electricity generation. While dead biomass microalgae are utilized for electron production in the anode chamber, the cathode chamber incorporates live and fresh microalgae. This integration introduces a dual-functionality where microalgae contribute not only to the generation of oxygen in the cathode but also serve as a substrate in the anode [40]. This innovative coupling of dead and live microalgae in different chambers of the MFC system adds complexity and versatility to its capabilities. In the cathode chamber of the M-MFC system, the introduction of live and fresh microalgae serves a dual purpose, showcasing a novel approach to enhance oxygen availability and electron acceptance (Table 2). The key function of the live microalgae lies in its ability to undergo photosynthesis, a crucial biological process that harnesses light energy to convert carbon dioxide and water into organic compounds and oxygen [3]. During photosynthesis, live microalgae absorb light energy through pigments such as chlorophyll. This energy is then utilized to drive a series of biochemical reactions, resulting in the production of carbohydrates and the release of oxygen. In the context of the M-MFC system, the oxygen generated through photosynthesis becomes a valuable asset in the cathode chamber. Firstly, the oxygen produced by live microalgae in the cathode chamber acts as an electron acceptor during the reduction reaction [6]. While conventional MFCs often employ expensive platinum-based materials as electron acceptors in the cathode, the M-MFC system leverages the naturally occurring oxygen produced by photosynthetic microalgae Table 2. This substitution not only reduces the reliance on costly materials but also aligns with the principles of sustainability by utilizing the inherent capabilities of live microorganisms. Moreover, the introduction of live microalgae in the cathode chamber provides a dynamic and self-sustaining mechanism [41]. As the microalgae continuously undergo photosynthesis, they contribute to the consistent generation of oxygen, creating a favorable environment for the reduction reaction to occur. Sustained oxygen production is essential for maintaining efficient electron flow within the M-MFC system, ensuring a steady and reliable bioelectricity generation process. The utilization of live microalgae in the cathode chamber not only addresses the need for an electron acceptor but also introduces a holistic approach to energy generation [8]. By integrating the natural photosynthetic capabilities of microalgae, the M-MFC system showcases a cost-effective and environmentally friendly alternative to traditional MFC cathodes, which often rely on expensive platinum-based catalysts. This innovative synergy between live microalgae and microbial processes demonstrates the potential for sustainable and economically viable MFC technology [37].
Comparison between Microbial Fuel Cells (MFCs) and Microalgae-Microbial Fuel Cells (M-MFCs).
Establishing an appropriate cathode configuration is a critical factor in enhancing bioelectricity generation and advancing MFC technology from pilot-scale applications to industrial-scale implementation. The cathode material plays a pivotal role in determining the power output of the MFC due to its high redox potential and efficient proton capture capabilities.
Common materials used for cathodes include carbon paper, fiber, granular graphite, copper (Cu), and platinum (Pt). Platinum, in particular, is employed in the cathode chamber to increase the reaction rate and reduce the activation energy of cathodic reactions in MFC. This innovative use of platinum has shown promise in improving MFC performance [42].
Initially, the use of platinum as a cathode material resulted in high electricity generation. However, over time, there was no significant difference in electricity generation between platinum and non-platinum cathodes, indicating a potential cost-saving opportunity. Given the high cost of platinum, various efforts have been made to explore alternatives and reduce the overall cost of MFC by substituting platinum with more economical materials.
4.3 The role of the microorganism
Exoelectrogens are a group of microorganisms, primarily bacteria, that are instrumental in the operation of MFCs. Their defining characteristic is their ability to release electrons as a byproduct of metabolizing organic compounds. This unique trait enables them to participate in the generation of electrical current within the MFC.
Exoelectrogens are typically situated in the anode chamber of the MFC, where they engage in the oxidation of organic substrates and transfer the resulting electrons to the anode electrode. This electron transfer process is at the heart of electricity production in MFCs [38].
This group of microorganisms is quite diverse, encompassing various species, including Geobacter, Shewanella, and Rhodoferax, among others. Each species may possess distinct attributes that impact their performance within MFCs. Exoelectrogens often form biofilms on the anode electrode’s surface. These biofilms act as conductive pathways, enhancing the efficiency of electron transfer by facilitating the movement of electrons to the anode [37].
Exoelectrogens display an ability to thrive in different environmental conditions, making them adaptable to various organic substrates and wastewater types. This adaptability is advantageous for MFC applications across diverse settings. Ongoing research endeavors focus on gaining a deeper understanding of exoelectrogens, their mechanisms of electron transfer, and methods to optimize their performance in MFCs. Genetic engineering and biofilm engineering are among the strategies explored to enhance the efficiency of electricity generation. Apart from their role in electricity generation, exoelectrogens also hold the potential for bioremediation by breaking down organic pollutants in wastewater.
While exoelectrogens show promise in MFCs, challenges remain, such as improving their metabolic rates and enhancing overall electron transfer efficiency. Researchers are actively investigating ways to enhance their performance and reduce the costs associated with MFC technology. Exoelectrogens are a critical component in the operation of MFCs, and ongoing research endeavors seek to unlock their potential for sustainable electricity generation and wastewater treatment [3].
5 Material design and configuration in M-MFCs
With or without the use of polymer electrolyte membranes, one or two chambers are frequently assembled in traditional MFC technology to produce electricity through a chemically specified substrate (such as a solution of glucose or acetate, for example) (PEM) [43, 44]. The two chambers namely an anode and cathode. In two-compartment MFCs, the anodic and cathodic chambers are joined in an H-shape, with an Ultrex or Nafion PEM salt bridge completing the circuit and maintaining the device’s electrical neutrality. Operationally, the cathodic chamber receives a continual oxygen supply while the anodic chamber is home to the organic substrate and sludge, with the exchange membrane supporting ionic transfer [45]. The anode, cathode, and membrane surface areas are crucial in determining how much electricity the MFC can produce. H-shaped two-compartment MFCs are mainly exclusively used in laboratory research due to their weak power densities and large internal resistances [46].
However, in the case of M-MFC, the cathode chamber is maintained by microalgal organisms, which necessitate the presence of carbon dioxide (CO2), water, and temperature for the purpose of growth and the production of fundamental nutrients. The cathode chamber receives the created carbon dioxide along with the anode electrolyte. Microalgae are biodegradable because they consume carbon dioxide, store it in their cells, and then release oxygen more quickly. After that, oxygen combines with the hydrogen ions (H+) that were produced at the anode and then travels through the PEM membrane to the cathode, where it forms fresh water.
Regarding the choice of material, anodic materials are recommended for their high conductivity, great chemical stability, and good biocompatibility [47]. Despite being benchmarked for its conductivity; copper’s antibacterial property makes it a less than ideal anodic material. A good substitute for copper used for anodic reasons in MFCs is stainless-steel mesh because it is non-corrosive and less hazardous. The remarkable flexibility and plasticity of carbon, which can be functionalized as crushed graphite plates, granules, rods, fibers, or glassy carbon, makes it one of the most ideal anodic possibilities [48].
Several variables, such as the number of electrons present, the kind of receiver, the presence of protons, the activity of the catalyst, and the electrode layout, can have an impact on the performance of the MFC cathode. The cathode chamber is sometimes referred as an anaerobic chamber, highlighting the crucial function of oxygen in it. Most scientists agree that O2 serves as an electron acceptor, drawing and consuming the electron produced in the anode chamber prior to interacting with the H+ that has migrated from the separating membrane [49]. Only water is produced as a result of such a procedure, demonstrating its advantageous impacts on the environment [50]. To enable oxygen replenishment and improve MFC activity, some designs position one side of the cathode in the cathode chamber whereas the other makes contact with air above the surface. The choice of cathode material is dependent on the material’s availability and oxidation potential. For a stabilized MFC performance, the non-toxicity of such chosen material as well as its oxygenated equivalents is also crucial.
An MFC’s usual structure consists of an electrode distance, a cationic membrane formed of ceramic or clayware material, and two chambers: an anode and a cathode [51]. To produce electricity in MFCs, depending on the properties of the electron acceptor, microorganisms’ biopotential, which is fuelled by metabolic and physiological activities, is responsible. The separation of the chambers while maintaining the anaerobic environment on the anodic side is made possible in large part by the membrane for proton exchange or other similarly functioning membranes [52]. In laboratory MFC research, Ultrex CMI-7000 membranes and Nafion are frequently used; the latter is more cost-effective. In the meantime, it verified that Polymer Inclusion Membranes (PIMs) based on ionic liquids are compatible with MFCs technology [53]. Porous clay materials, including such innovative porous clay earthenware (NCE), showed better power outputs when employed as a separator in comparison to such Polymer Electrolyte Membranes (PEM) [33]. Ionic liquid had a beneficial role in moving the activated proton through the separator, as evidenced by the observed positive association between the quantity of immobilized ionic liquid membranes and the overall power production of MFCs.
6 Current issues and limitations related to M-MFC system
The relationship between microalgae and bacteria is not limited to particular species, making it challenging to identify unique metabolites for each species. In a combined system, identifying and choosing microalgal strains with specific biochemical compositions is essential for estimating the potential for power generation and recovering valuable products [54].
Many electroactive microorganisms have not been cultured yet, making it difficult to understand their metabolic processes and how they cycle nutrients. In the cathode chamber, when microalgal cells grow too much, they block light, which slows down their own growth. The changes in metabolism and the interactions between bacteria and microalgae over time would alter the in situ conditions of the culture [54].
Sometimes, when microalgae and bacteria are grown together, the microalgae can increase the pH and salinity of the culture, which might slow down the growth of electroactive bacteria. It’s better to use a mix of different microbes in the anode chamber of MFCs because they are more resilient to stress and can adapt to different nutrients. These MFCs work by using reactions in the mitochondria of microalgae cells. If we improve the ability of microalgae to turn sunlight into energy through genetic changes, it can help overcome some limitations. Overall, the ability of microalgae and bacteria to handle stress in these MFCs can lead to more electricity and biomass for making biofuels [55].
Using ion-exchange membranes or separators like PEM poses practical limitations for the widespread adoption of microalgal-assisted MFCs technology due to its high cost and increased internal resistance. PEM is commonly used in MFC because it offers relatively good conductivity to cations and low internal resistance compared to other separators. PEM membranes can be categorized based on materials or pore size, including Cation Exchange Membranes (CEM), Anion Exchange Membranes (AEM), Bipolar Membranes (BPM), Microfiltration Membranes (MFM), and Ultrafiltration Membranes (UFM). Nafion and Ultrex membranes are popular choices in MFC systems due to their excellent proton selectivity. Additionally, PEM membranes can transport cations such as Na+, K+, NH4+, Ca2+, and Mg2+. However, finding an efficient membrane material at a lower cost remains a challenge for scaling up MFCs [56].
Some studies have explored cost-effective alternatives to traditional membranes in MFCs, such as using materials like glass fiber or removing the membrane altogether. Low-cost ceramic materials, including clayware and coconut shells, have shown promise in enhancing power generation by improving proton movement and biofilm thickness. While membrane-less microalgal-assisted MFCs have been investigated, they tend to exhibit lower power densities due to challenges in electron and proton transfer. Moving forward, focusing on suitable membrane configurations, utilizing low-cost materials as separators, and enhancing the electrochemical activity of microorganisms through genetic modifications hold potential for scaling up microalgal-assisted MFCs and improving their efficiency [56].
However, the M-MFC system is not without challenges. Despite the enhanced electron generation, power output levels may still face limitations, potentially impacting the system’s suitability for high-demand electricity applications. Environmental sensitivity, particularly in maintaining the optimal conditions for the microbial community, could present operational challenges [14].
The commercial adoption and establishment of standardized practices for M-MFCs are still evolving, presenting challenges regarding technology maturity and widespread adoption. Spatial constraints for larger M-MFC systems, coupled with the initial investment and technical expertise required, may hinder their feasibility, particularly in urban or space-limited environments.
To sum up, M-MFCs show significant promise for sustainable energy generation and environmental applications. However, addressing challenges related to power output, environmental sensitivity, technology maturity, and spatial constraints is essential to fully realize the potential of this innovative technology. Ongoing research efforts are vital for overcoming these limitations and advancing the effectiveness of Microalgae-assisted Microbial Fuel Cells.
7 Conclusions and remarks
In conclusion, this study unveils the innovative potential of integrating live and dead microalgae within MFCs to achieve a symbiotic synergy with far-reaching implications. The dual-chamber approach, leveraging dead microalgae in the anode and live microalgae in the cathode, has demonstrated remarkable outcomes in sustainable bioelectricity generation and environmental applications.
The findings underscore the significance of deceased microalgae biomass as a valuable substrate, enhancing microbial metabolism and electron generation in the anode chamber. Simultaneously, live microalgae, through their photosynthetic activity in the cathode chamber, contribute to efficient oxygen production, thereby facilitating the reduction reaction and sustaining electron flow within the M-MFC system.
The integration of microalgae not only advances bioelectricity generation but also extends the functionality of the M-MFC system to wastewater treatment. Initial findings indicate a promising capability for wastewater treatment, highlighting the flexibility of this integrated method beyond traditional MFC models.
The environmental impact of the M-MFC system is evident, as it diminishes reliance on traditional electron acceptors while presenting opportunities for electricity generation and environmental benefits. The inventive incorporation of both live and deceased microalgae in MFCs paves the way for additional exploration in sustainable energy and ecological revitalization.
As scientists strive to address the overlapping issues of energy and the environment, the collaborative synergy showcased in this study marks a transformative moment in MFC technology. This research not only represents a significant advancement in comprehending the potential of Microalgae-assisted MFCs but also catalyzes future initiatives in sustainable bioenergy and environmental endeavors.
In summary, the incorporation of both living and dead microalgae into Microbial Fuel Cells emerges as a hopeful pathway, offering opportunities for progress that resonate with the overarching objectives of establishing sustainable and versatile energy systems.
Acknowledgments
The authors would like to thank the Universiti Malaysia Pahang Al-Sultan Abdullah (UMPSA) for allowing us the opportunity to submit this manuscript.
References
- Nagarajan D., Lee D.J., Kondo A., Chang J.S. (2017) Recent insights into biohydrogen production by microalgae – from biophotolysis to dark fermentation, Bioresource Technol. 227, 373–387. https://doi.org/10.1016/j.biortech.2016.12.104. [CrossRef] [Google Scholar]
- Zabed H.M., Akter S., Yun J., Zhang G., Zhang Y., Qi X. (2020) Biogas from microalgae: technologies, challenges and opportunities, Renew. Sustain. Energy Rev. 117, July, 109503. https://doi.org/10.1016/j.rser.2019.109503. [CrossRef] [Google Scholar]
- Elshobary M.E., Zabed H.M., Yun J., Zhang G., Qi X. (2021) Recent insights into microalgae-assisted microbial fuel cells for generating sustainable bioelectricity, Int. J. Hydrogen Energy 46, 4, 3135–3159. https://doi.org/10.1016/j.ijhydene.2020.06.251. [CrossRef] [Google Scholar]
- Kosourov S., Murukesan G., Seibert M., Allahverdiyeva Y. (2017) Evaluation of light energy to H2 energy conversion efficiency in thin films of cyanobacteria and green alga under photoautotrophic conditions, Algal Res. 28, September, 253–263. https://doi.org/10.1016/j.algal.2017.09.027. [CrossRef] [Google Scholar]
- Oyekale J., Petrollese M., Tola V., Cau G. (2020) Impacts of renewable energy resources on effectiveness of grid-integrated systems: succinct review of current challenges and potential solution strategies, Energies 13, 18, 4856. https://doi.org/10.3390/en13184856. [CrossRef] [Google Scholar]
- Shukla M., Kumar S. (2018) Algal growth in photosynthetic algal microbial fuel cell and its subsequent utilization for biofuels, Renew. Sustain. Energy Rev. 82, October, 402–414. https://doi.org/10.1016/j.rser.2017.09.067. [CrossRef] [Google Scholar]
- Zabed H.M., Akter S., Yun J., Zhang G., Awad F.N., Qi X., Sahu J.N. (2019) Recent advances in biological pretreatment of microalgae and lignocellulosic biomass for biofuel production, Renew. Sustain. Energy Rev. 105, January, 105–128. https://doi.org/10.1016/j.rser.2019.01.048. [CrossRef] [Google Scholar]
- Arun S., Sinharoy A., Pakshirajan K., Lens P.N.L. (2020) Algae based microbial fuel cells for wastewater treatment and recovery of value-added products, Renew. Sustain. Energ. Rev. 132, 110041. [CrossRef] [Google Scholar]
- Jiang K., Baburin I.A., Han P., Yang C., Fu X., Yao Y., Li J., Cánovas E., Seifert G., Chen J., Bonn M., Feng X., Zhuang X. (2020) Interfacial approach toward benzene-bridged polypyrrole film–based micro-supercapacitors with ultrahigh volumetric power density, Adv. Funct. Mater. 30, 7, 1–9. https://doi.org/10.1002/adfm.201908243. [Google Scholar]
- Ren L., Sun X., Zhang L., Huang H., Zhao Q. (2020) Exergy analysis for docosahexaenoic acid production by fermentation and strain improvement by adaptive laboratory evolution for Schizochytrium sp, Bioresource Technol. 298, 122562. https://doi.org/10.1016/j.biortech.2019.122562. [CrossRef] [Google Scholar]
- Li M., Zhou M., Tan C., Tian X. (2019) Enhancement of CO2 biofixation and bioenergy generation using a novel airlift type photosynthetic microbial fuel cell, Bioresour. Technol. 272 October, 501–509. [CrossRef] [Google Scholar]
- Greenman J., Gajda I., Ieropoulos I. (2019) Microbial fuel cells (MFC) and microalgae; photo microbial fuel cell (PMFC) as complete recycling machines, Sustain. Energ. Fuels 3, 2546–2560. [CrossRef] [Google Scholar]
- Jaiswal K.K., Kumar V., Vlaskin M.S., Sharma N., Rautela I., Nanda M., Arora N., Singh A., Chauhan P.K. (2020) Microalgae fuel cell for wastewater treatment: recent advances and challenges, J. Water Process Eng. 38, 101549. [CrossRef] [Google Scholar]
- Sharma M., Salama E.-S., Zhang P., Zhang L., Xing X., Yue J., Song Z., Nan L., Yujun S., Li X. (2022) Microalgae-assisted microbial fuel cells for electricity generation coupled with wastewater treatment: biotechnological prospective, J. Water Process Eng. 49, 102966. https://doi.org/10.1016/j.jwpe.2022.102966. [CrossRef] [Google Scholar]
- Reddy C.N., Kakarla R., Min B. (2018) Chapter 3.7 – Algal biocathodes, in: S.V. Mohan, A. Pandey, S. Varjani (eds), Biomass, biofuels, biochemicals: microbial electrochemical technology: sustainable platform for fuels, chemicals and remediation, Elsevier, pp. 525–547. https://doi.org/10.1016/B978-0-444-64052-9.00021-2. [Google Scholar]
- Symes M.D., Cogdell R.J., Cronin L. (2013) Designing artificial photosynthetic devices using hybrid organic–inorganic modules based on polyoxometalates, Phil. Trans. R. Soc. A. 3712011041120110411. http://doi.org/10.1098/rsta.2011.0411. [Google Scholar]
- Nwoba E.G., Parlevliet D.A., Laird D.W., Alameh K., Moheimani N.R. (2019) Light management technologies for increasing algal photobioreactor efficiency, Algal Res. 39, 9264. https://doi.org/10.1016/j.algal.2019.101433. [Google Scholar]
- Meylani V., Surahman E., Fudholi A., Almalki W.H., Ilyas N., Sayyed R.Z. (2023) Biodiversity in microbial fuel cells: review of a promising technology for wastewater treatment, J. Environ. Chem. Eng. 11, 2, 109503. https://doi.org/10.1016/j.jece.2023.109503. [CrossRef] [Google Scholar]
- Patwardhan S.B., Savla N., Pandit S., Gupta P.K., Mathuriya A.S., Lahiri D., Jadhav D.A., Rai A.K., KanuPriya Ray R.R., Singh V. (2021) Microbial fuel cell united with other existing technologies for enhanced power generation and efficient wastewater treatment, Appl. Sci. 11, 22, 10777. https://doi.org/10.3390/app112210777. [CrossRef] [Google Scholar]
- Baicha Z., Salar-García M.J., Ortiz-Martínez V.M., Hernández-Fernández F.J., de los Ríos A.P., Labjar N., Lotfi E., Elmahi M. (2016) A critical review on microalgae as an alternative source for bioenergy production: A promising low cost substrate for microbial fuel cells, Fuel Process. Technol. 154, 104–116. https://doi.org/10.1016/j.fuproc.2016.08.017. [CrossRef] [Google Scholar]
- Gude V.G. (2016) 8 – Microbial fuel cells for wastewater treatment and energy generation, in: Scott K., Yu E.H. (eds), Microbial electrochemical and fuel cells, Woodhead Publishing, pp. 247–285. ISBN 9781782423751, https://doi.org/10.1016/B978-1-78242-375-1.00008-3. [CrossRef] [Google Scholar]
- Kusmayadi A., Leong Y.K., Yen H.W., Huang C.Y., Dong C.D., Chang J.S. (2020) Microalgae-microbial fuel cell (mMFC): an integrated process for electricity generation, wastewater treatment, CO2 sequestration and biomass production, Int. J. Energy Res. 44, 12, 9254–9265. https://doi.org/10.1016/j.chemosphere.2021.129800. [CrossRef] [Google Scholar]
- Slate A.J., Whitehead K.A., Brownson D.A.C., Banks C.E. (2019) Microbial fuel cells: An overview of current technology, Renew. Sustain. Energy Rev 101, 60–81. https://doi.org/10.1016/j.rser.2018.09.044. [CrossRef] [Google Scholar]
- Saravanan A., Senthil Kumar P., Srinivasan S., Jeevanantham S., Kamalesh R., Karishma S. (2022) Sustainable strategy on microbial fuel cell to treat the wastewater for the production of green energy, Chemosphere 290, 133295. https://doi.org/10.1016/j.chemosphere.2021.133295. [CrossRef] [PubMed] [Google Scholar]
- Hoang A.T., Nižetić S., Ng K.H., Papadopoulos A.M., Le A.T., Kumar S., Hadiyanto H., Pham V.V. (2022) Microbial fuel cells for bioelectricity production from waste as sustainable prospect of future energy sector, Chemosphere 287, 3, 132285. https://doi.org/10.1016/j.chemosphere.2021.132285. [CrossRef] [PubMed] [Google Scholar]
- Khandaker S., Das S., Hossain T., Islam A., Miah M.R., Awual R. (2021) Sustainable approach for wastewater treatment using microbial fuel cells and green energy generation – a comprehensive review, J. Mole. Liq. 344, 117795. https://doi.org/10.1016/j.molliq.2021.117795. [CrossRef] [Google Scholar]
- Shuiliang C., Patil S.A., Schröder U. (2018) Substrate crossover effect and performance regeneration of the biofouled rotating air-cathode in microbial fuel cell, Front. Energy Res. 6, 85. https://doi.org/10.3389/fenrg.2018.00085. [CrossRef] [Google Scholar]
- Ahmed S.F., Mofijur M., Islam N., Parisa T.A., Rafa N., Bokhari A., Klemeš J.J., Mahlia T.M.I. (2022) Insights into the development of microbial fuel cells for generating biohydrogen, bioelectricity, and treating wastewater, Energy 254, 124163. https://doi.org/10.1016/j.energy.2022.124163. [CrossRef] [Google Scholar]
- Yahampath Arachchige Don C.D.Y., Babel S. (2021) Circulation of anodic effluent to the cathode chamber for subsequent treatment of wastewater in photosynthetic microbial fuel cell with generation of bioelectricity and algal biomass, Chemosphere 278, 130455. https://doi.org/10.1016/j.chemosphere.2021.130455. [CrossRef] [PubMed] [Google Scholar]
- Sarma P.J., Malakar B., Mohanty K. (2024) Self-sustaining bioelectricity generation in Plant-based Microbial Fuel Cells (PMFCs) with microalgae-assisted oxygen-reducing biocathode, Biomass Conv. Bioref 14, 15, 16973–16986. https://doi.org/10.1007/s13399-023-03848-z. [CrossRef] [Google Scholar]
- Zhang X., Li X., Zhao X., Li Y. (2019) Factors affecting the efficiency of a bioelectrochemical system: a review, RSC Adv. 9, 34, 19748–19761. https://doi.org/10.1039/c9ra03605a [CrossRef] [Google Scholar]
- Palanisamy G., Jung H.-Y., Sadhasivam T., Kurkuri M.D., Kim S.C., Roh S.-H. (2019) A comprehensive review on microbial fuel cell technologies: Processes, utilization, and advanced developments in electrodes and membranes, J Cleaner Prod. 221, 598–621. https://doi.org/10.1016/j.jclepro.2019.02.172. [CrossRef] [Google Scholar]
- Daud S.M., Daud W.R.W., Bakar M.H.A., Kim B.H., Somalu M.R., Muchtar A., Jahim J.M., Muhammed Ali S.A. (2020) Low-cost novel clay earthenware as separator in microbial electrochemical technology for power output improvement, Bioproc. Biosyst. Eng. 43, 8, 1369–1379. https://doi.org/10.1007/s00449-020-02331-7. [CrossRef] [PubMed] [Google Scholar]
- Yadav G., Sharma I., Ghangrekar M., Sen R. (2020) A live bio-cathode to enhance power output steered by bacteria-microalgae synergistic metabolism in microbial fuel cell, J. Power Sources 449, 227560. https://doi.org/10.1016/j.jpowsour.2019.227560. [CrossRef] [Google Scholar]
- Chaturvedi A., Kundu P.P. (2021) Recent advances and perspectives in platinum-free cathode catalysts in microbial fuel cells, J. Environ. Chem. Eng. 9, 4, 105662. https://doi.org/10.1016/j.jece.2021.105662. [CrossRef] [Google Scholar]
- Hay J.X.W., Wu T.Y., Juan J.C., Jahim J. Md. (2017) Effect of adding brewery wastewater to pulp and paper mill effluent to enhance the photofermentation process: wastewater characteristics, biohydrogen production, overall performance, and kinetic modeling, Environ. Sci. Poll. Res. 24, 11, 10354–10363. https://doi.org/10.1007/s11356-017-8557-9. [CrossRef] [PubMed] [Google Scholar]
- Ou S., Kashima H., Aaron D.S., Regan J.M., Mench M.M. (2017) Full cell simulation and the evaluation of the buffer system on air-cathode microbial fuel cell, J. Power Sources 347, 159–169. https://doi.org/10.1016/j.jpowsour.2017.02.031. [CrossRef] [Google Scholar]
- Ndayisenga F., Yu Z., Yu Y., Lay C.-H., Zhou D. (2018) Bioelectricity generation using microalgal biomass as electron donor in a bio-anode microbial fuel cell, Bioresour Technol. 270, 2018, 286–293. https://doi.org/10.1016/j.biortech.2018.09.052. [CrossRef] [Google Scholar]
- Rahman M.M., Asiri A.M., Khaleque M.A., Sheikh M.C. (2019) Introducing an alternate conjugated material for enhanced lead(II) capturing from wastewater, J. Clean. Prod. 224, 920–929. [CrossRef] [Google Scholar]
- Mehrotra S., Kumar V.K., Gajalakshmi S., Pathak B. (2021) Bioelectrogenesis from ceramic membrane-based algal-microbial fuel cells treating dairy industry wastewater, Sustain. Energy Technol. Assess. 48, 101653. https://doi.org/10.1016/j.seta.2021.101653. [Google Scholar]
- Enamala M.K., Dixit R., Tangellapally A., Singh M., Dinakarrao S.M., Chavali M., Pamanji S.R., Ashokkumar V., Kadier A., Chandrasekhar K. (2020) Photosynthetic microorganisms (Algae) mediated bioelectricity generation in microbial fuel cell: concise review, Environ. Technol. Innov. 19, 100959. https://doi.org/10.1016/j.eti.2020.100959. [CrossRef] [Google Scholar]
- Asiri A.M., Rahman M.M. (2020) Optimization of an innovative composited material for effective monitoring and removal of cobalt (II) from wastewater, J. Mol. Liq. 298, 112035. [CrossRef] [Google Scholar]
- Allam F., Elnouby M., El-Khatib K.M., El-Badan D.E., Sabry S.A. (2020) Water hyacinth (Eichhornia crassipes) biochar as an alternative cathode electrocatalyst in an air cathode single chamber microbial fuel cell, Int. J. Hydrogen Energy 45, 10, 5911–5927. https://doi.org/10.1016/j.ijhydene.2019.09.164. [CrossRef] [Google Scholar]
- Selvaraj D., Somanathan A., Jeyakumar R.B., Kumar G. (2020) Generation of electricity by the degradation of electro-Fenton pretreated latex wastewater using double chamber microbial fuel cell, Int. J. Energy Res. 44, 15, 12496–12505. https://doi.org/10.1002/er.5503. [CrossRef] [Google Scholar]
- Senthilkumar K., Anappara S., Krishnan H., Ramasamy P. (2020) Simultaneous power generation and Congo red dye degradation in double chamber microbial fuel cell using spent carbon electrodes, Energy Sources A Recovery Util. Environ. Eff. 1–17. https://doi.org/10.1080/15567036.2020.1781978. [Google Scholar]
- Zhu Z., Zhang Y.H.P. (2017) In vitro metabolic engineering of bioelectricity generation by the complete oxidation of glucose, metabolic engineering 39, September, 110–116. https://doi.org/10.1016/j.ymben.2016.11.002. [CrossRef] [PubMed] [Google Scholar]
- Halim M.A., Rahman M.O., Eti I.A., Shefa N.R., Ibrahim M., Alam M.J. (2020) Electricity generation in different cell connections with optimized anodic materials in microbial fuel cells, Energy Sources A Recovery Util. Environ. Eff. 1–13. https://doi.org/10.1080/15567036.2020.1851818. [Google Scholar]
- Goswami R., Mishra V.K. (2018) A review of design, operational conditions and applications of microbial fuel cells, Biofuels 9, 2, 203–220. https://doi.org/10.1080/17597269.2017.1302682. [CrossRef] [Google Scholar]
- Wang H., Wang Q., Li X., Wang Y., Jin P., Zheng Y., Huang J., Li Qingbiao (2019) Bioelectricity generation from the decolorization of reactive blue 19 by using microbial fuel cell, J. Environ. Manage. 248, July, 109310. https://doi.org/10.1016/j.jenvman.2019.109310. [CrossRef] [Google Scholar]
- Wang S., Jiang J., Zhao Q., Wang K. (2022) Effects of substrate type on variation of sludge organic compounds, bioelectric production and microbial community structure in bioelectrochemically-assisted sludge treatment wetland, J. Environ. Manage. 307, December, 114548. https://doi.org/10.1016/j.jenvman.2022.114548. [CrossRef] [Google Scholar]
- Yousefi V., Mohebbi-Kalhori D., Samimi A. (2017) Ceramic-based microbial fuel cells (MFCs): a review, Int. J. Hydrogen Energy 42, 3, 1672–1690. https://doi.org/10.1016/j.ijhydene.2016.06.054. [CrossRef] [Google Scholar]
- Das I., Das S., Ghangrekar M.M. (2020) Application of bimetallic low-cost CuZn as oxygen reduction cathode catalyst in lab-scale and field-scale microbial fuel cell, Chem. Phys. Lett. 751, April, 137536. https://doi.org/10.1016/j.cplett.2020.137536. [CrossRef] [Google Scholar]
- Ortiz-Martínez V.M., Gajda I., Salar-García M.J., Greenman J., Hernández-Fernández F.J., Ieropoulos I. (2016) Study of the effects of ionic liquid-modified cathodes and ceramic separators on MFC performance, Chem. Eng. J. 291, 317–324. https://doi.org/10.1016/j.cej.2016.01.084. [CrossRef] [Google Scholar]
- Lu M., Chen S., Babanova S., Phadke S., Salvacion M., Mirhosseini A., Chan S., Carpenter K., Cortese R., Bretschger O. (2017) Long-term performance of a 20-L continuous flow microbial fuel cell for treatment of brewery wastewater, J. Power Sourc. 356, 274–287. [CrossRef] [Google Scholar]
- Salama E.-S., Govindwar S.P., Khandare R.V., Roh H.S., Jeon B.H., Li X. (2019) Can omics approaches improve microalgal biofuels under abiotic stress?, Trends Plant Sci. 24, 611624. [Google Scholar]
- Munoz-Cupa C., Hu Y., Xu C., Bassi A. (2021) An overview of microbial fuel cell usage in wastewater treatment, resource recovery and energy production, Sci. Total Environ. 754, 142429. https://doi.org/10.1016/j.scitotenv.2020.142429. [CrossRef] [Google Scholar]
All Tables
Comparison between Microbial Fuel Cells (MFCs) and Microalgae-Microbial Fuel Cells (M-MFCs).
All Figures
 |
Fig. 1 Schematic of a typically employed two-chamber microbial fuel cell highlighting the various electrochemical and electro-microbiological processes. Figure adapted from reference [23]. |
| In the text | |
 |
Fig. 2 Microalgae-Microbial Fuel Cell (M-MFC). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.




