| Issue |
Sci. Tech. Energ. Transition
Volume 78, 2023
Selected Papers from First European Conference on Gas Hydrates (ECGH), 2022
|
|
|---|---|---|
| Article Number | 36 | |
| Number of page(s) | 14 | |
| DOI | https://doi.org/10.2516/stet/2023034 | |
| Published online | 06 December 2023 | |
Regular Article
Investigating cyclopentane hydrate nucleation and growth using microfluidics
1
IFP Energies nouvelles, 1-4 Avenue de Bois-Préau, 92852 Rueil-Malmaison, France
2
Navier, Ecole des Ponts, Université Gustave Eiffel, CNRS, Marne-la-Vallée, France
* Corresponding author: anne.sinquin@ifpen.fr
Received:
11
May
2023
Accepted:
6
November
2023
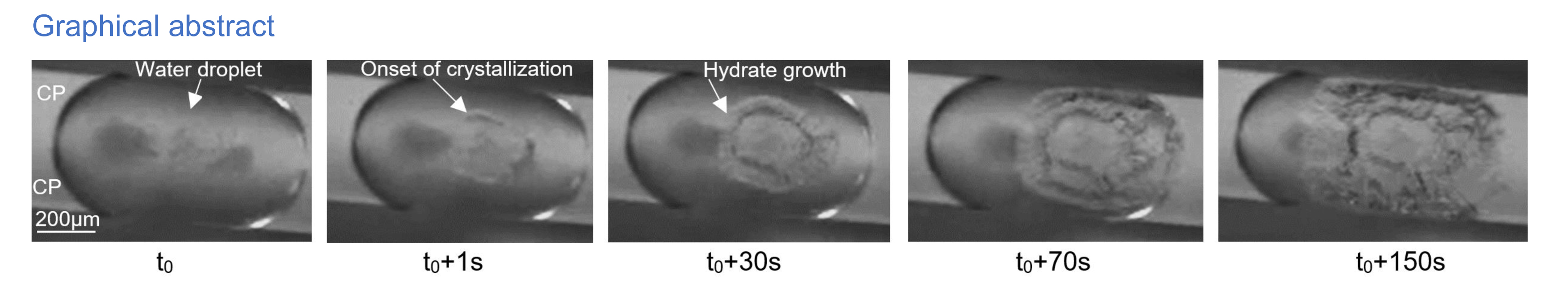
The success of geological storage of carbon dioxide (CO2) in depleted oil and gas reservoirs relies among other aspects on the efficiency of CO2 injection, especially in the near-wellbore area where flow rates are high. CO2 hydrates pressure/temperature equilibrium conditions may be reached in this zone due to cooling associated with the Joule–Thomson effect; such CO2 hydrate formation may lead to strong injectivity loss and impair drastically the onsite well operations. In this study, cyclopentane hydrates (CPH) were employed as CO2 hydrate proxy (i.e. formation at atmospheric pressure) to mimic CO2 hydrate formation at higher pressure. In this study, the nucleation and growth processes were determined using a droplet-based in-house-microfluidic device. The generation of water droplets in cyclopentane liquid using the co-flow method was achieved. Trains of identical water droplets were stored in a serpentine channel. Each isolated droplet in this channel serves as a separate reactor. The temperature was controlled using a Peltier module to initiate hydrate nucleation at low temperatures. The isolated droplets provided the opportunity to statistically analyze the kinetic behaviors by varying key parameters, such as thermal history and water salinity. Detection of the onset of crystallization in water droplets over time and temperature allowed us to plot conversion curves based on imposed parameters. The effect of thermal history and dissociation temperature was first compared using pure water. This study marks the initial investigation into how NaCl influences CPH formation in microfluidic devices, focusing on isolated water droplets within serpentine tubes. The progression of ice nucleation, ice melting, the onset of CPH crystallization, CPH growth, and CPH dissociation are illustrated in water droplets exposed to changing temperatures. The addition of NaCl in the water during the procedure exhibited a noteworthy impact on CPH formations. With the same temperature profile, salt concentration delays nucleation (thermodynamic effect) and slows down growth. Our findings suggest that higher subcooling accelerates nucleation and growth rates. Initial lateral growth rates ranged from 4.22 μm/s to 2.14 μm/s, with a subcooling of 4.2 °C observed between 2 and 7 min for a pure water droplet.
Key words: Cyclopentane hydrates / Nucleation / Crystallization kinetics / Microfluidic device / Salinity effect / CO2 storage / Depleted reservoir
© The Author(s), published by EDP Sciences, 2023
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.