| Numéro |
Sci. Tech. Energ. Transition
Volume 79, 2024
|
|
|---|---|---|
| Numéro d'article | 1 | |
| Nombre de pages | 10 | |
| DOI | https://doi.org/10.2516/stet/2023042 | |
| Publié en ligne | 9 janvier 2024 | |
Regular Article
An applied study on energy analysis of a coke oven
1
Directorate of Coke Facilities, Kardemir Iron and Steel Co., Karabuk, Turkey
2
Energy Systems Engineering Department, Karabuk University, Karabuk, Turkey
* Corresponding author: selcukselimli@karabuk.edu.tr
Received:
18
October
2023
Accepted:
19
December
2023
In this study, the energy view of an oven of a 70-oven coke battery in an iron and steel plant was evaluated based on operating parameters and recommendations for improving efficiency were made. A mass and energy balance per coking period (p) was created for a coke oven. It was found that during each coking period, 51.2% of the energy input was used as coking heat. It is predicted that approximately 6.91% of the input energy can be recovered from flue gas into the combustion air. By recovering the heat from the flue gas into the combustion air, the efficiency of the coke oven can be increased to 58.11%. The heat of the coke oven gas can be recovered and converted into usable form, which accounts for 6.53% of the total energy input. With the dry quenching process, it is possible to recover around 24% of the energy used from coke. Improved oven insulation, heat recovery from coke and flue gases, and the dry quenching process can recover energy worth more than 25.19 GJ/p. The energy efficiency of the furnace was predicted to rise to 82.11% with coke dry quenching and to more than 88.64% with coke gas heat recovery and insulation upgrades. The potential economic savings are $2578, equivalent to a reduction in CO₂ emissions of 2.45 tons per coking period. The financial equivalent of emissions reductions from carbon trading could be $233 per coking period. Through the processes of dry coke quenching, coke gas (CG), and flue gas heat recovery and thermal insulation improvements of the coke battery, the total amount of recoverable energy can exceed 617,294 GJ/year.
Key words: Coke oven / Energy / Efficiency / Saving / Emission
© The Author(s), published by EDP Sciences, 2024
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
1 Practical applications
The main objective of this study is to raise industry awareness of potential energy recovery opportunities and system improvements by conducting an energy analysis within the operating parameters of the coking plant of an integrated iron and steel plant. Furthermore, the number of applied studies in literature is rather small. The indirect contribution of this study is to create additional resources for young researchers and engineers new to the industry and to raise awareness among iron and steel investors of the level of returns that can be achieved by investing in energy-intensive equipment. A mass and energy balance of a coke oven was performed, the performance of the oven during operation was evaluated, and recommendations were made for possible energy recovery and system upgrades.
2 Introduction
A large part of the world’s iron and steel production comes from China and India. In 2019, Turkey accounted for 1.8% of global production and ranked eighth with 33.7 million tons of production [1]. The steel industry consumes a lot of energy because it requires numerous melting, cooling, heating, and solidification processes [2]. The annual share of steel production in global energy consumption and CO₂ emissions is 5% and 4%, respectively [3]. In Turkey, the sectoral shares of energy consumption and emissions of the steel industry are 25% and 40%, respectively [4, 5]. Turkey’s production is expected to increase significantly by 2030 [6]. The proportion of energy consumption and CO₂ emissions is expected to increase dramatically as production increases. A key to increasing industry awareness is identifying potential savings and system improvements in operational facilities through energy analysis. The by-products of the steel industry include tar, coke oven gas, blast furnace gas (BFG), etc. Significant energy savings can be achieved by evaluating these products. Improving system efficiency can be achieved through both heat recovery and heat loss prevention [7]. Turkey can cut its energy consumption by at least 20% (45 million tons of oil) by implementing regular and effective energy efficiency measures [8]. Increasing the energy efficiency of the industry will increase competitiveness, secure energy supplies, and mitigate climate change [9]. The iron and steel production process consists of numerous subunits that carry out a series of operations from raw materials to semi-finished steel products. Coking plants, sinter plants, blast furnaces, steel workshops, rolling mills, and power generation plants are some of the most important subunits of integrated iron and steel plants. Furthermore, contingent on product requirements and corporate policies, there might be intermediate production facilities. One of the primary energy sources for the iron and steel industry is metallurgical coke, which is produced by heating coal to temperatures above 1100 °C in oxygen-free coke ovens [10, 11]. This process results in the release of volatiles in the coal through a series of reactions, leaving behind high-carbon coke that contains no volatiles [12]. A coking plant is connected to an integrated steel production network [13]. Coal preparation, batteries, crushing and screening plants, and by-product plants form the four main subunits of coking plants built to supply metallurgical coke to blast furnaces. The coking plants produce metallurgical coke, cast coke, walnut coke, coke powder and gas, raw tar, raw benzene, naphthalene, and ammonium sulfate.
3 Introduce of the coking plant
To make it easier for practitioners to understand the energy relationships and loss points of the coking plant, the production conditions of a specific coking plant were examined, and the data collected was evaluated. There are three different coke batteries in the iron and steel plant where this study is conducted. The facility comprises three batteries: 1–2 batteries with 44 ovens, 3–4 batteries with 56 ovens, and 5–6 batteries with 70 ovens. The oven is approximately 4.5 m high, 0.45 m wide and 13.59 m deep. The coal capacity of the oven for one cooking period is approximately 20 tons. The coking temperature ranges from 1200 °C to 1300 °C, and the coking period is nearly eighteen hours. An energy analysis was performed on 5–6 batteries with 70 ovens. Coke gas at 2820 Nm³/h and blast furnace gas at 35,200 Nm³/h are supplied to battery ovens as fuel. Figure 1a and 1b shows the top and side views of the coke oven that is the subject of this paper.
 |
Figure 1 Coke battery image; (a) top view and (b) side view. |
Figure 2 illustrates the coke oven material flow diagram.
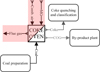 |
Figure 2 Coke oven material flow diagram. |
In a coking plant, the coke oven is where coal is coked. Figure 2 illustrates how the reaction energy released during the coking process supports the process, while the heat released by the reaction of the mixed gas (MG) fed to the combustion chamber and the combustion air ensures the coking of the coal in the coke chamber.
4 Literature review
Few recent studies have been published in the literature that assess a coking plant’s energy analysis with its operating conditions. The following is a summary of some of them. In a study conducted by Larsson et al., an assessment was made of the connections and interrelations among coking plants and other associated facilities in terms of mass and energy. It was asserted that the material and energy costs allocated to the various production units within a steel production facility are roughly 28–30% for the coke oven plant, 56–59% for the blast furnace, and 14% for the basic oxygen furnace [14]. Approximately 59.5% of the heat applied to the coke oven was applied to coal to produce coking, according to a study on energy analyses conducted by Ertem and Ozdabak on a coking plant [15]. The research conducted by Sultanguzin et al. examined the production of metallurgical coke, the coal fuel-energy balance, and the optimization of steel production based on energy and environmental standards [16]. The energy efficiency of an industrial furnace was studied by Ajah et al. They recommended some ways to make the furnace more energy-efficient. They claim that increasing the temperature of the combustion air, recovering energy from the flue gas, and improving the insulation properties of the refractory wall increase the energy efficiency of the furnace from 57% to 78% [17]. As demonstrated by Nogami et al. examined the material and energy balances of an integrated iron and steel facility that includes a blast furnace, hot stove, coke oven, and coke dry quenching. They provided diagrams to illustrate the mass and energy flow boundary conditions for a coke oven in their study [18]. Zhao et al. attempted to optimize the control of coke gas in real time using an echo states network system based on Gaussian processes in order to support energy optimization in a practical study [19]. The temperature distribution and heat transfer of the coke chamber, heating wall, and combustion chamber of a coke oven were examined numerically by Xiao et al. using the multi-compartment coupling mathematical model that was developed. According to the data, using regular silica bricks could result in a 60-minute reduction in coking time and a 49% decrease in fuel gas consumption during a single cycle [20]. Liu and Guo claimed that coking consumes between 40% and 50% of an oven’s energy input [21]. The research that Casemiro et al. carried out an energy analysis study to ascertain the coke oven’s potential for heat recovery. According to their analysis, there was an energy loss of 3.47 GJ per unit ton of coke produced [22]. In their numerical investigation, Lin et al. created numerical models to look into the coke chamber’s gas flow, volatile evolution, and temperature profile [23]. The research that Magrinho et al. carried out an energy analysis study using the ECLIPSE software to provide a coking plant’s environmental impact and energy requirements characterization and optimization [24]. Mahato et al. attempted to do an optimization study on a coke oven to lower the gas consumption of the coke oven and boost coke production more effectively. They successfully reduced the cycle time and the amount of coke oven gas consumed after conducting a number of studies [25]. Buczynski et al. numerically assessed the energy performance of an industrial coking plant by using a one-dimensional time-dependent mathematical model. The moving boundary conditions were taken into consideration in the study in order to establish the system energy balance [26]. In their numerical study, Neto et al. used Aspen Plus software to simulate and examine the impact of a coke oven battery’s combustion chamber temperature on fuel consumption. They claim that a new set of regulations can save the amount of gas used in coke ovens by 12% [27]. A numerical study conducted by Veinskii developed a couple of equations for the energy balance of a coke oven as well as the material balance of the coking process. Therefore, exergetic efficiency was determined at 57.78% for the oven [28]. Das et al. created predictive models to characterize the thermo-physical properties of the oven charge by taking the chemical composition and structural factors into account [29]. Tiwari et al. examined the coking process’s rate of heat utilization. The coal cake’s heat penetration rate was found to be 60% [30]. This study assessed the energy performance of a coke oven in operation at a coking plant. Coking plants are divisions of iron and steel production plants, and there are a few recent practical studies assessing the energy performance of these plants in the literature.
Global economic issues, wars, and power struggles following COVID-19, and notable increases in energy costs have resulted in an industrial contraction and increased difficulty for commercial competition. To obtain an advantage in product pricing in the market, it is crucial to minimize production costs. In an energy-intensive iron and steel facility, this study made recommendations for improvement and cost savings by analyzing the energy savings that can be achieved through system upgrades and investments. The study seeks to mitigate the effects of the current economic challenges on the production sector in this regard. The study’s widespread effects include establishing a resource for young researchers and engineers who are just starting out in the iron and steel industry and raising awareness among investors about the amount of money that can be saved by making investments in energy-intensive facilities. The method and content of the study are intended to contribute to the literature as well as the iron and steel industry.
5 The coke oven’s thermal characteristics
This study determines the mass and energy balance of a coke oven and assesses the oven’s performance in its current configuration. Improvements to the system and prospects for energy recovery were suggested. Table 1 shows the lower heating value (LHV) and chemical composition of the MG fed into the combustion chamber.
Chemical composition of fuel burned in the coke oven.
Table 1 illustrates that the MG’s LHV is roughly equivalent to 3161 kJ/Nm³. The combustion reaction in the coke oven of MG takes place with an air–fuel ratio (AFR) of 9.74.
6 Governing equations
Equation (1) can be used to establish the general mass balance.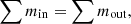 (1)where the symbols for the inlet and outlet mass are
(1)where the symbols for the inlet and outlet mass are 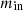 and
and 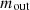 . Equations (2) and (3) can be used to determine the mass balance for the combustion chamber and coke oven [31].
. Equations (2) and (3) can be used to determine the mass balance for the combustion chamber and coke oven [31].
Equation (2) provides the mass balance for the coke oven.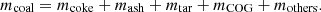 (2)
(2)
In equation (2), 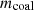 is the mass of fed coal to the oven;
is the mass of fed coal to the oven; 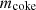 is the mass of coke;
is the mass of coke; 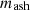 is the mass of ash;
is the mass of ash; 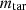 is the mass of tar;
is the mass of tar; 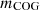 is the mass of COG; and
is the mass of COG; and 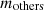 is the mass of volatile gases; etc. gained per coking period. Equation (3) can be used to calculate the mass balance of the combustion chamber.
is the mass of volatile gases; etc. gained per coking period. Equation (3) can be used to calculate the mass balance of the combustion chamber.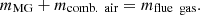 (3)
(3)
In equation (3), the mass input of the MG and combustion air into the combustion chamber is represented by 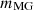 and
and  , respectively. The amount of flue gas released during a coking period is represented by
, respectively. The amount of flue gas released during a coking period is represented by 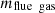 .
.
The energy balance general equation is denoted by equation (4). (4)
(4)
The input and output energies are expressed by 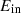 and
and  in equation (4), respectively. Equation (5) can be used to represent energy balance.
in equation (4), respectively. Equation (5) can be used to represent energy balance.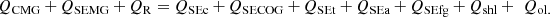 (5)
(5)
In equation (5), the combustion energy of MG is represented as 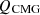 , the sensible energy of MG as
, the sensible energy of MG as 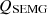 , the reaction energy as
, the reaction energy as 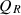 , the sensible energy of coke as
, the sensible energy of coke as 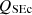 , the sensible energy of COG as
, the sensible energy of COG as 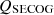 , the sensible energy of tar as
, the sensible energy of tar as 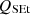 , the sensible energy of ash as
, the sensible energy of ash as 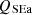 , the sensible energy of flue gas as
, the sensible energy of flue gas as 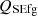 , the surface heat loss by convection (Q
cv
) and radiation (Q
r
) as
, the surface heat loss by convection (Q
cv
) and radiation (Q
r
) as 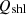 and other losses such as openness, leakages, etc. are designated as
and other losses such as openness, leakages, etc. are designated as 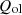 . Equations (6)–(8) can be used to estimate the surface heat loss from the surfaces of the coke oven [15, 32].
. Equations (6)–(8) can be used to estimate the surface heat loss from the surfaces of the coke oven [15, 32].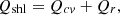 (6)
(6)
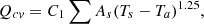 (7)
(7)
![$$ {Q}_r={C}_2{C}_3\sum {A}_s\left[{\left(\frac{{T}_s}{100}\right)}^4-{\left(\frac{{T}_a}{100}\right)}^4\right]. $$](/articles/stet/full_html/2024/01/stet20230198/stet20230198-eq31.gif) (8)
(8)
The variables in equations (7) and (8) are 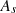 ,
, 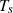 , and
, and 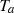 , which represent the surface area, surface, and ambient temperatures, respectively. Also,
, which represent the surface area, surface, and ambient temperatures, respectively. Also, 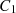 ,
, 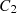 and C
3 are constant values.
and C
3 are constant values.
7 Energetic view of the coke oven
Equations (6)–(8) were used to compute the surface heat losses of the coke oven by applying the average surface temperatures recorded by the oven thermal camera system, which is depicted in Figure 3.
 |
Figure 3 Oven surface temperature distribution captured by a thermal camera with estimated thermal losses. |
The upper section of the oven has the highest loss rate based on the heat losses shown in Figure 3. Despite the equal upper and lower surface areas of the oven, the temperature differences cause a difference in heat loss of about 1,034,892 kJ/p. The rates of heat loss from the pusher and the coke sides are fairly similar. There is 2,234,842 kJ/p in total thermal loss from the surfaces. 98.26% of the surface heat loss is lost by convection or about 2,195,989 kJ/p, and 1.74% is lost by radiation, or 38,853 kJ/p. The combustion chamber and coke oven mass balances are shown in Figure 4.
 |
Figure 4 Mass balance for the coke oven. |
As can be seen in Figure 4, to coke the 18,625 kg of coal loaded into the coke oven during coking period, 12,044 kg of MG and 117,308 kg of air were burned in the combustion chamber. Flue gas emissions totaling 129,352 kg were released by combustion. Upon completion of the coking of 18,625 kg of coal, the following products were produced: 11,994 kg of coke, 2720 kg of coke oven gas, 1415 kg of tar, 1490 kg of ash, 1006 kg of volatile gases, etc. were acquired. As shown in Figure 5, energy balance was attained for the coke oven.
 |
Figure 5 Coke oven energy balance. |
According to the energy balance shown in Figure 5 for the coke oven, burning the MG in the combustion chamber produces approximately 38,080,500 kJ/p of combustion energy. With an ambient temperature of 20 °C as a reference, the MG fed to the oven has a temperature of 60 °C and delivers 886,899 kJ/p sensible heat to the combustion chamber. Being at the same temperature as the surrounding air, the combustion air that is fed into the oven is considered to have zero sensible energy. At high temperatures, the coal that is put into the oven transforms into coke, coke oven gas, tar, ash, and minute amounts of other volatiles. Coal reacts during the coking process, releasing some heat. The difference in the heating value of coal and the carbonaceous products that are produced during the process like coke, coke oven gas, and tar, is the amount of heat energy that is released during the reaction. The reaction energy was calculated to be 28,345,043 kJ/p. 56.57% of the energy entering the oven during the coking process comes from the mixed gas’s combustion, 42.11% comes from the reaction, and 1.32% comes from the mixed gas’s sensible energy. During the coking process, 34,459,318 kJ/p, or 51.2% of the energy entering the coke oven, is applied to the coal; however, 32,853,124 kJ/p, or 48.8% of the energy, escapes the oven as flue gas, surface heat losses, and other losses (openness, leakage, etc.). Figure 6 provides a visual summary of the mass and energy balance of the coke oven. Coal, coke, coke gas, and blast furnace gas heating values are shown in Figure 6 were supplied by the facility laboratories. Thermophysical characteristics of combustion air, tar, ash, and flue gas were calculated using relations and evaluations from papers written by Eisermann et al. [33], Eremin et al. [34], Fardhyanti and Damayanti [35], Ratnaningsih et al. [36], Lesniak et al. [37], Cengel and Boles [38].
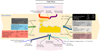 |
Figure 6 The coke oven’s mass and energy balance. |
Coke, tar, and ash exit the oven at 1100 °C, and coke oven gas exits the oven at 850 °C at the end of the coking process. As these products are taken out of the coke battery and allowed to cool to room temperature, the thermal energy that was gained during coking is lost to ambient. A proportional summary of the current oven’s energy relationship can be found in Figure 7. Furthermore, a sizable amount of the energy input is lost to the environment due to the release of flue gas into the atmosphere, hot coke, and the cooling of the coke gas to ambient conditions. The estimates for the recoverability of these losses are also displayed in Figure 7. The energy lost during coking can be partially recovered with three distinct investments. The first option is to use a preheater to heat the combustion air using flue gas heat. The second approach involves recovering into use the thermal load of the hot coke by cooling it to the appropriate temperature inside the dry quenching unit that will be installed.
 |
Figure 7 The proportional energy diagram of the coke oven. |
Lastly, an economizer can be used to recover a portion of the heat from the coke gas, which can then be used to heat areas, plant showers, or require hot water to process.
High-pressure water is sprayed to cool the hot coke. There is a loss of thermal energy due to the steam that is released into the atmosphere during cooling. According to a study by Takagi, 83% of the coke’s sensible heat can be recovered by cooling the coke using the dry quenching method after the coking process is complete [39]. By implementing a dry quenching system in the current coke battery, it appears feasible to retrieve around 16,149,234 kJ/p of energy per oven. This amount is equivalent to about 24% of the energy input of the coke oven. Several studies reported in the literature claim that by recovering the sensible energy of coke oven gas into another fluid via a heat exchanger, it can be transformed into a form that can be used. Additionally, it states that lowering the temperature of the coke oven gas below 450 °C could result in tar condensation inside the heat exchanger and lower efficiency [40]. In a heat exchanger, approximately 4,392,256 kJ/p of heat recovery can be achieved by transferring the heat of the coke oven gas at 850 °C to another fluid until it cools to 450 °C. This amount is equivalent to 6.53% of the energy entering the coke oven and 48.19% of the sensible energy of the coke oven gas. About 200 °C is the temperature of flue gas, and before it is released into the atmosphere, some of its thermal energy can be recovered. Zhang and Chowdhury [41] and Zhang et al. [42] stated that by attaching a radial heat pipe heat exchanger to the coke oven flue gas line, hot water steam can be produced using the sensible heat of the flue gas. They claimed that in this manner, 17.31% of the sensible heat from the flue gas could be recovered [41, 42]. An energy recovery of 4,654,182 kJ/p can be achieved by using the flue gas heat from the coke oven with a heat exchanger to create steam or warm the combustion air. The combustion air was not preheated and had an average temperature of 20 °C during the study period. Recovering flue gas heat into the combustion air lowers the pressure on the environment from thermal pollution while increasing process energy efficiency. Achieving 58.11% input energy utilization can be possible through the recovery of flue gas heat into the combustion air. The recovery rate can be equal to 6.91% of the energy input. By substituting high-insulating alternative refractory materials for the precast and fireclay brick used in coke oven doors, walls, and roofs, heat loss from surfaces can be reduced. By recovery recommendations and minimizing heat loss for the coke oven under study, it is estimated that energy savings of more than 25,195,672 kJ/p can be realized. This approximate value represents 37.44% of the energy input for the coking process in the coke oven. The coke oven can save up to 8,818,485,200 kJ by performing 350 coking cycles on average per year. One kilogram of coal contains 70–80% carbon, and burning it releases 2.93 kg of CO₂, according to a study conducted by Jennifer et al. [43]. Turkey uses natural gas with a calorific value of 40,062 kJ/m³, according to data from BOTAŞ Petroleum Pipelines Co. 2020 [44]. Natural gas is priced at $4.1/m³. For an oven, the potential economic size of recovery estimated amounts to $902,496 annually. A reduction in carbon emissions of 859,153 kg per year could result from this saving, according to the evaluation. The savings’ annual carbon trade can be equivalent price is roughly $81,619. The 70 ovens in the coke battery could result in total annual savings of more than 617,294 GJ if the potential investment is made. For the coke battery, the economic value of the gain could be $63,174,720 annually. The reduction of carbon emissions can be roughly 60.14 kt/year, and the gains in carbon trade value can be $5,713,330/year for the coke battery.
8 Statement of significance
Based on the boundary conditions of a coke oven in the coke batteries of an integrated iron and steel plant, this study determined the mass and energy balance and identified savings potential. Due to its methodology and content, this study is an applied field study. Although raising the sector’s awareness of potential savings is the study’s primary goal, its broader impact is also can be assessed as providing a current resource for upcoming engineers and researchers in the field.
9 Conclusion
A single oven of a coke battery with 70 ovens is the subject of an energy analysis in this study. With the coke oven’s operating conditions in mind, calculations and assessments were carried out. The study was carried out for a coking period of 18 h. The results showed that 51.2% of the input energy was found to be applied to the coal during the coking process, and 48.8% was lost because of flue gas, heat transfer from surfaces, and other ways like leaks and openness. As things stand, cooling at the end of coking process causes releasing the sensible energy of the final products into the atmosphere. An approximate recovery of 16,149,234 kJ/p sensible heat of coke per oven is anticipated with the installation of a dry coke quenching unit in the coking plant. This corresponds to 24% of the energy used for coking. It was found that by using a heat exchanger, 4,392,256 kJ/p or 6.53% of the energy supplied could be recovered from the sensible heat of the coke oven gas. By preheating the oven combustion air using flue gas heat, 4,654,182 kcal/p, or 6.91% of energy input, can be saved and the oven energy efficiency can reach 58.11%. By using refractory materials with high thermal insulation properties for walls, roofs, and coke oven doors, thermal insulation can be improved, and losses reduced. Achieving energy savings of 25.19 GJ is possible by recovering the heat from coke, coke oven gas, and flue gas for the per coking process. It was estimated that the furnace energy efficiency could increase to 82.11% with coke dry quenching and over 88.64% with coke gas heat recovery and insulation improvements. It is predicted that the coke battery could save more than 617,294 GJ/year. The projected recovery could result in an energy price equivalent of $63,174,720, a $5,713,330 carbon trading equivalent, and a 60.14 kt annual emissions reduction contribution.
Data availability statement
No data, models, or code were generated or used during the study.
Acknowledgments
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- World Steel Association, Global Crude Steel Output Increases by 3.4% in 2019. Available at: https://www.worldsteel.org/media-centre/press-releases/2020/Global-crude-steel-output-increases-by-3.4–in-2019.html. [Google Scholar]
- Choudhury R., Bhaktavatsalam A.K. (1997) Energy inefficiency of Indian steel industry – scope for energy conservation. Energy Convers. Manag. 38, 2, 167–171. https://doi.org/10.1016/0196-8904(96)00029-5. [CrossRef] [Google Scholar]
- Xu C., Cang D. (2010) A brief overview of low CO₂ emission technologies for iron and steel making. J. Iron Steel Res. Int. 17, 3, 1–7. https://doi.org/10.1016/S1006-706X(10)60064-7. [CrossRef] [Google Scholar]
- Olmez G.M., Dilek F.B., Karanfil T., Yetis U. (2016) The environmental impacts of iron and steel industry: a life cycle assessment study. J. Clean. Prod. 130, 195–201. https://doi.org/10.1016/j.jclepro.2015.09.139. [CrossRef] [Google Scholar]
- Akbostancı E., Tunc G.I., Turut-Asık S. (2011) CO₂ emissions of Turkish manufacturing industry: a decomposition analysis. Appl. Energy 88, 6, 2273–2278. https://doi.org/10.1016/j.apenergy.2010.12.076. [CrossRef] [Google Scholar]
- Ates S.A. (2015) Energy efficiency and CO₂ mitigation potential of the Turkish iron and steel industry using the LEAP (long-range energy alternatives planning) system. Energy 90, 417–428. https://doi.org/10.1016/j.energy.2015.07.059. [CrossRef] [Google Scholar]
- Adanir T., Ozdemir T. Energy and exergy analyses of the power plant at Eregli iron and steel factory, Global Conference on Global Warming 2008, July 6–10, 2008, Istanbul, Turkey, pp. 168. [Google Scholar]
- Terzi U.K., Baykal R. (2011) Efficient and effective use of energy: a case study of TOFAS. Environ. Res. Eng. Manag. 1, 55, 29–33. [Google Scholar]
- Tutunoglu Y., Guven A., Ozturk I.T. (2012) Energy analysis of glass tempering furnace. Eng. Mach. 53, 629, 55–62. [Google Scholar]
- Yu W., Thurston G.D. (2023) An interrupted time series analysis of the cardiovascular health benefits of a coal coking operation closure. Environ. Res. Health 1, 4, 45002. https://doi.org/10.1088/2752-5309/ace4ea. [Google Scholar]
- Cui B., Wu B., Wang M., Jin X., Shen Y., Chang L. (2024) A preliminary study on the quality evaluation of coking coal from its structure thermal transformation: applications of fluidity and swelling indices. Fuel 355, 129418. https://doi.org/10.1016/j.fuel.2023.129418. [CrossRef] [Google Scholar]
- Mayo S., Sakurovs R., Jenkins D., Mahoney M. (2023) Using xenon K-edge subtraction to image the gas-accessible porosity distribution within metallurgical cokes and their partially reacted products. Tomo. Mater. Struct. 3, 100013. https://doi.org/10.1016/j.tmater.2023.100013. [Google Scholar]
- Razzaq R., Li C., Zhang S. (2013) Coke oven gas: availability, properties, purification, and utilization in China. Fuel 113, 287–299. https://doi.org/10.1016/j.fuel.2013.05.070. [CrossRef] [Google Scholar]
- Larsson M., Sandberg P., Dahl J., Soderstrom M., Vourinen H. (2004) System gains from widening the system boundaries: analysis of the material and energy balance during renovation of a coke oven battery. Int. J. Energy Res. 28, 1051–1064. https://doi.org/10.1002/er.1013. [CrossRef] [Google Scholar]
- Ertem M.E., Ozdabak A. (2005) Energy balance application for Erdemir Coke Plant with thermal camera measurements. Appl. Therm. Eng. 25, 423–433. https://doi.org/10.1016/j.applthermaleng.2004.05.013. [CrossRef] [Google Scholar]
- Sultanguzin I.A., Isaev M.V., Kurzanov S.Y. (2011) Optimizing the production of coke, coal chemicals, and steel on the basis of environmental and energy criteria. Metallurgist 54, 9–10, 600–607. [CrossRef] [Google Scholar]
- Ajah S.A., Idorenyin D., Ezurike B.O., Nwokenkwo U., Ikwuagwu C.V. (2023) Thermal analysis to investigate the effects of operating parameters on conventional cupola furnace efficiency. Proc. Inst. Mech. Eng. Part E 237, 4, 1354–1366. https://doi.org/10.1177/09544089221113401. [CrossRef] [Google Scholar]
- Nogami H., Yagi J., Kitamura S., Austin P.R. (2006) Analysis on material and energy balances of ironmaking systems on blast furnace operations with metallic charging, top gas recycling and natural gas injection. ISIJ Int. 46, 12, 1759–1766. [CrossRef] [Google Scholar]
- Zhao J., Liu Q., Wang W., Pedrycz W., Cong L. (2012) Hybrid neural prediction and optimized adjustment for coke oven gas system in steel industry. IEEE Trans. Neural Netw. Learn. Syst. 23, 3, 439–450. https://doi.org/10.1109/TNNLS.2011.2179309. [CrossRef] [PubMed] [Google Scholar]
- Xiao K., Wang Y., Hu H., Wen Z., Lou G., Su F., Dou R., Liu X. (2023) Numerical analysis on heat transfer process in the coke oven with the multi-chamber coupling mathematical model. Case Stud. Therm. Eng. 44, 102858. https://doi.org/10.1016/j.csite.2023.102858. [CrossRef] [Google Scholar]
- Liu H., Guo W. (2023) Comparative study on life cycle energy consumption, carbon emissions and economic performance of various coke-oven gas utilization schemes. Fuel 332, 125706. https://doi.org/10.1016/j.fuel.2022.125706. [CrossRef] [Google Scholar]
- Casemiro R.L., Tapanes N.C.O., Souza M.C.L., Santana A.I.C., Pinto W.C.L. (2022) Energetic estimation of heat-recovery coke oven, Eng. Térm. (Therm. Eng.) 21, 2, 13–20. [Google Scholar]
- Lin W., Feng Y., Zhang X. (2015) Numerical study of volatiles production, fluid flow and heat transfer in coke ovens. Appl. Therm. Eng. 81, 353–358. https://doi.org/10.1016/j.applthermaleng.2015.02.056. [CrossRef] [Google Scholar]
- Magrinho A., Semiao V., Carvalho M.G. (2001) Energy and environmental analysis of an entire coke production plant using ECLIPSE. Int. J. Energy Res. 25, 93–106. [CrossRef] [Google Scholar]
- Mahato N., Agarwal H., Jain J. (2022) Reduction of specific heat consumption by modification of reversal cycle period of coke oven battery. Mater. Today: Proc. 61, 1149–1153. https://doi.org/10.1016/j.matpr.2021.12.032. [CrossRef] [Google Scholar]
- Buczynski R., Weber R., Kim R., Schwöppe P. (2016) One-dimensional model of heat-recovery, non-recovery coke ovens. Part II: Coking-bed sub-model. Fuel 181, 1115–1131. https://doi.org/10.1016/j.fuel.2016.01.086. [CrossRef] [Google Scholar]
- Farias Neto G.W., Leite M., Marcelino T., Carneiro L.O., Brito K.D., Brito R.P. (2021) Optimizing the coke oven process by adjusting the temperature of the combustion chambers. Energy 217, 119419. https://doi.org/10.1016/j.energy.2020.119419. [CrossRef] [Google Scholar]
- Veinskii V.V. (2011) Simulation of coking in coke furnaces. Coke Chem. 54, 12, 469–476. https://doi.org/10.3103/S1068364X11120167. [CrossRef] [Google Scholar]
- Das S.K., Godiwalla K.M., Mehrotra S.P. (2007) A mathematical model for prediction of physical properties of the coke oven charge during carbonisation. High Temp. Mater. Process. 26, 1, 43–57. [Google Scholar]
- Tiwari H.P., Saxena V.K., Haldar S.K., Sriramoju S.K. (2017) Assessment of thermal efficiency of heat recovery coke making. Heat Mass Transf. 53, 8, 2517–2529. https://doi.org/10.1007/s00231-017-2003-x. [CrossRef] [Google Scholar]
- Larsson M. (2004) Process integration in the steel industry: Possibilities to analyse energy use and environmental impacts for an integrated steel mill, Doctoral dissertation, Lulea University of Technology. [Google Scholar]
- Lu J., Lu X., Liu H., Wang H., He H. (2010) Calculation and analysis of dissipation heat loss in large-scale circulating fluidized bed boilers. Appl. Therm. Eng. 30, 13, 1839–1844. [CrossRef] [Google Scholar]
- Eisermann W., Johnson P., Conger W.L. (1980) Estimating thermodynamic properties of coal, char, tar and ash. Fuel Process. Technol. 3, 1, 39–53. https://doi.org/10.1016/0378-3820(80)90022-3. [CrossRef] [Google Scholar]
- Eremin A.Y., Mishchikhin V.G., Stakheev S.G., Gilyazetdinov R.R., Shvetsov V.I. (2011) Using coke-battery flue gas to dry coal batch before coking. Coke Chem. 54, 3, 77–85. https://doi.org/10.3103/S1068364X11030021. [CrossRef] [Google Scholar]
- Fardhyanti D.S., Damayanti A. (2016) Analysis of coal tar compositions produced from sub-bituminous kalimantan coal tar. J. Sains Teknol. 14, 1, 31–38. https://doi.org/10.15294/sainteknol.v14i1.7613. [Google Scholar]
- Ratnaningsih W., Sukandar, Wahyuningrum D. (2021) Coal tar waste utilization by cracking into fuel source and its separation using the fractional vacuum distillation method. IOP Conf. Ser.: Earth Environ. Sci. 802, 1, 12039. https://doi.org/10.1088/1755-1315/802/1/012039. [CrossRef] [Google Scholar]
- Lesniak B., Slupik L., Jakubina G. (2013) The determination of the specific heat capacity of coal based on literature data. Chemik 67, 6, 560–571. [Google Scholar]
- Cengel Y.A., Boles M.A., Kanoglu M. (2019) Thermodynamics: an engineering approach, McGraw Hill, New York ISBN 9789813157873. [Google Scholar]
- Takagi S. (1980) Energy saving in ironmaking processes. Trans. ISIJ 20, 338–352. [CrossRef] [Google Scholar]
- Bisio G., Rubatto G. (2000) Energy saving and some environment improvements in coke-oven plants. Energy 25, 3, 247–265. https://doi.org/10.1016/S0360-5442(99)00066-3. [CrossRef] [Google Scholar]
- Zhang H., Chowdhury A.J.K. (2019) Research on integrated technology of desulphurization, denitration and waste heat recovery of coke oven flue gas. Nat. Environ. Pollut. Technol. 18, 5, 1621–1625. [Google Scholar]
- Zhang G., Li S., Jiang H., Xie G. (2015) Application of radial heat pipe to heat recovery of flue gas, in: 5th International Conference on Advanced Engineering Materials and Technology (AEMT 2015), August 22–23, 2015, Guangzhou, China, pp. 282–285. [Google Scholar]
- O'Keefe J.M., Henke K.R., Hower J.C., Engle M.A., Stracher G.B., Stucker J.D., Drew J.W., Staggs W.D., Murray T.M., Hammond M.L. III, Adkins K.D. (2010) CO2, CO, and Hg emissions from the Truman Shepherd and Ruth Mullins coal fires, eastern Kentucky, USA. Sci. Total Environ. 408, 7, 1628–1633. [CrossRef] [Google Scholar]
- Türkmen B.A. (2022) Environmental performance of high-efficiency natural gas combined cycle plant. Energy Sources A: Recovery Util. Environ. Eff. 44, 1, 57–74. https://doi.org/10.1080/15567036.2020.1856974. [CrossRef] [Google Scholar]
All Tables
All Figures
 |
Figure 1 Coke battery image; (a) top view and (b) side view. |
| In the text | |
 |
Figure 2 Coke oven material flow diagram. |
| In the text | |
 |
Figure 3 Oven surface temperature distribution captured by a thermal camera with estimated thermal losses. |
| In the text | |
 |
Figure 4 Mass balance for the coke oven. |
| In the text | |
 |
Figure 5 Coke oven energy balance. |
| In the text | |
 |
Figure 6 The coke oven’s mass and energy balance. |
| In the text | |
 |
Figure 7 The proportional energy diagram of the coke oven. |
| In the text | |
Les statistiques affichées correspondent au cumul d'une part des vues des résumés de l'article et d'autre part des vues et téléchargements de l'article plein-texte (PDF, Full-HTML, ePub... selon les formats disponibles) sur la platefome Vision4Press.
Les statistiques sont disponibles avec un délai de 48 à 96 heures et sont mises à jour quotidiennement en semaine.
Le chargement des statistiques peut être long.




